The amygdala and basal forebrain as a pathway for motivationally guided attention
- PMID: 25297102
- PMCID: PMC4188973
- DOI: 10.1523/JNEUROSCI.2106-14.2014
The amygdala and basal forebrain as a pathway for motivationally guided attention
Abstract
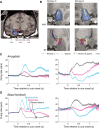
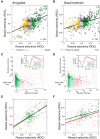
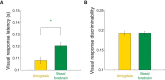
Visual stimuli associated with rewards attract spatial attention. Neurophysiological mechanisms that mediate this process must register both the motivational significance and location of visual stimuli. Recent neurophysiological evidence indicates that the amygdala encodes information about both of these parameters. Furthermore, the firing rate of amygdala neurons predicts the allocation of spatial attention. One neural pathway through which the amygdala might influence attention involves the intimate and bidirectional connections between the amygdala and basal forebrain (BF), a brain area long implicated in attention. Neurons in the rhesus monkey amygdala and BF were therefore recorded simultaneously while subjects performed a detection task in which the stimulus-reward associations of visual stimuli modulated spatial attention. Neurons in BF were spatially selective for reward-predictive stimuli, much like the amygdala. The onset of reward-predictive signals in each brain area suggested different routes of processing for reward-predictive stimuli appearing in the ipsilateral and contralateral fields. Moreover, neurons in the amygdala, but not BF, tracked trial-to-trial fluctuations in spatial attention. These results suggest that the amygdala and BF could play distinct yet inter-related roles in influencing attention elicited by reward-predictive stimuli.
Keywords: amygdala; attention; basal forebrain; emotion.
Copyright © 2014 the authors 0270-6474/14/3413757-11$15.00/0.
Figures


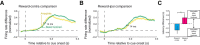
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
