Inhibition of adaptive immunity by IL9 can be disrupted to achieve rapid T-cell sensitization and rejection of progressive tumor challenges
- PMID: 25297635
- PMCID: PMC4254354
- DOI: 10.1158/0008-5472.CAN-14-0836
Inhibition of adaptive immunity by IL9 can be disrupted to achieve rapid T-cell sensitization and rejection of progressive tumor challenges
Abstract
The tolerogenic cytokine IL9 promotes T regulatory cell function and allergic airway inflammation, but it has not been extensively studied in cancer. In this report, we used IL9-deficient mice to investigate the effects of IL9 in multiple models of breast and colon cancer development. Eliminating endogenous IL9 enabled sensitization of host T cells to tumors, leading to their early rejection without the requirement of vaccines or immunomodulatory therapies. Notably, IL9-deficient mice acquired immunologic memory, which actively protected from residual disease and tumor rechallenge, an effect linked to activation of CD8(+) T cells. Depletion of either CD8(+) or CD4(+) T cells abolished the benefits of IL9 loss to tumor control. Adoptive transfer experiments showed that T cells from tumor-rejecting IL9-deficient mice retained their effector competency in wild-type animals. Moreover, neutralizing IL9 antibody phenocopied the effects of IL9 gene deletion by slowing tumor progression in wild-type animals. Our results show the ability of IL9 to function as an inhibitor of adaptive immunity that prevents the formation of immunologic memory to a growing tumor, highlighting the potential for IL9 neutralization as a unique tool for cancer immunotherapy.
©2014 American Association for Cancer Research.
Conflict of interest statement
Conflict of interest: The authors declare that they have no conflict of interest.
Figures


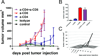
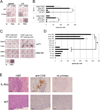
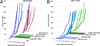
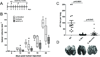
References
-
- Stassen M, Schmitt E, Bopp T. From interleukin-9 to T helper 9 cells. Ann N Y Acad Sci. 1247:56–68. - PubMed
-
- Visekruna A, Ritter J, Scholz T, Campos L, Guralnik A, Poncette L, et al. Tc9 cells, a new subset of CD8(+) T cells, support Th2-mediated airway inflammation. Eur J Immunol. 43:606–618. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

