Spider mites suppress tomato defenses downstream of jasmonate and salicylate independently of hormonal crosstalk
- PMID: 25297722
- PMCID: PMC4301184
- DOI: 10.1111/nph.13075
Spider mites suppress tomato defenses downstream of jasmonate and salicylate independently of hormonal crosstalk
Abstract
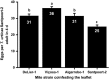
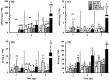
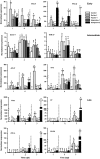
Plants respond to herbivory by mounting a defense. Some plant-eating spider mites (Tetranychus spp.) have adapted to plant defenses to maintain a high reproductive performance. From natural populations we selected three spider mite strains from two species, Tetranychus urticae and Tetranychus evansi, that can suppress plant defenses, using a fourth defense-inducing strain as a benchmark, to assess to which extent these strains suppress defenses differently. We characterized timing and magnitude of phytohormone accumulation and defense-gene expression, and determined if mites that cannot suppress defenses benefit from sharing a leaf with suppressors. The nonsuppressor strain induced a mixture of jasmonate- (JA) and salicylate (SA)-dependent defenses. Induced defense genes separated into three groups: 'early' (expression peak at 1 d postinfestation (dpi)); 'intermediate' (4 dpi); and 'late', whose expression increased until the leaf died. The T. evansi strains suppressed genes from all three groups, but the T. urticae strain only suppressed the late ones. Suppression occurred downstream of JA and SA accumulation, independently of the JA-SA antagonism, and was powerful enough to boost the reproductive performance of nonsuppressors up to 45%. Our results show that suppressing defenses not only brings benefits but, within herbivore communities, can also generate a considerable ecological cost when promoting the population growth of a competitor.
Keywords: Solanum lycopersicum (tomato); Tetranychus spp. (spider mite); defense suppression; herbivore communities; hormonal crosstalk; jasmonic acid (JA); salicylic acid (SA).
© 2014 The Authors. New Phytologist © 2014 New Phytologist Trust.
Figures

References
-
- Alba JM, Glas JJ, Schimmel BCJ, Kant MR. Avoidance and suppression of plant defenses by herbivores and pathogens. Journal of Plant Interactions. 2011;6:1–7.
-
- Ament K, Krasikov V, Allmann S, Rep M, Takken FLW, Schuurink RC. Methyl salicylate production in tomato affects biotic interactions. Plant Journal. 2010;62:124–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

