Ketamine coadministration attenuates morphine tolerance and leads to increased brain concentrations of both drugs in the rat
- PMID: 25297798
- PMCID: PMC4439876
- DOI: 10.1111/bph.12974
Ketamine coadministration attenuates morphine tolerance and leads to increased brain concentrations of both drugs in the rat
Abstract
Background and purpose: The effects of ketamine in attenuating morphine tolerance have been suggested to result from a pharmacodynamic interaction. We studied whether ketamine might increase brain morphine concentrations in acute coadministration, in morphine tolerance and morphine withdrawal.
Experimental approach: Morphine minipumps (6 mg·day(-1) ) induced tolerance during 5 days in Sprague-Dawley rats, after which s.c. ketamine (10 mg·kg(-1) ) was administered. Tail flick, hot plate and rotarod tests were used for behavioural testing. Serum levels and whole tissue brain and liver concentrations of morphine, morphine-3-glucuronide, ketamine and norketamine were measured using HPLC-tandem mass spectrometry.
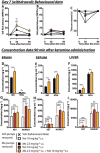
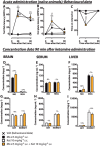
Key results: In morphine-naïve rats, ketamine caused no antinociception whereas in morphine-tolerant rats there was significant antinociception (57% maximum possible effect in the tail flick test 90 min after administration) lasting up to 150 min. In the brain of morphine-tolerant ketamine-treated rats, the morphine, ketamine and norketamine concentrations were 2.1-, 1.4- and 3.4-fold, respectively, compared with the rats treated with morphine or ketamine only. In the liver of morphine-tolerant ketamine-treated rats, ketamine concentration was sixfold compared with morphine-naïve rats. After a 2 day morphine withdrawal period, smaller but parallel concentration changes were observed. In acute coadministration, ketamine increased the brain morphine concentration by 20%, but no increase in ketamine concentrations or increased antinociception was observed.
Conclusions and implications: The ability of ketamine to induce antinociception in rats made tolerant to morphine may also be due to increased brain concentrations of morphine, ketamine and norketamine. The relevance of these findings needs to be assessed in humans.
© 2014 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures



References
-
- Amphoux A, Vialou V, Drescher E, Brüss M, La Cour CM, Rochat C, et al. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–952. - PubMed
-
- Arroyo-Novoa CM, Figueroa-Ramos MI, Miaskowski C, Padilla G, Paul SM, Rodríguez-Ortiz P, et al. Efficacy of small doses of ketamine with morphine to decrease procedural pain responses during open wound care. Clin J Pain. 2011;27:561–566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

