The PRO-ACT database: design, initial analyses, and predictive features
- PMID: 25298304
- PMCID: PMC4239834
- DOI: 10.1212/WNL.0000000000000951
The PRO-ACT database: design, initial analyses, and predictive features
Abstract
Objective: To pool data from completed amyotrophic lateral sclerosis (ALS) clinical trials and create an open-access resource that enables greater understanding of the phenotype and biology of ALS.
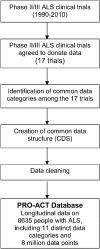
Methods: Clinical trials data were pooled from 16 completed phase II/III ALS clinical trials and one observational study. Over 8 million de-identified longitudinally collected data points from over 8,600 individuals with ALS were standardized across trials and merged to create the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) database. This database includes demographics, family histories, and longitudinal clinical and laboratory data. Mixed effects models were used to describe the rate of disease progression measured by the Revised ALS Functional Rating Scale (ALSFRS-R) and vital capacity (VC). Cox regression models were used to describe survival data. Implementing Bonferroni correction, the critical p value for 15 different tests was p = 0.003.
Results: The ALSFRS-R rate of decline was 1.02 (±2.3) points per month and the VC rate of decline was 2.24% of predicted (±6.9) per month. Higher levels of uric acid at trial entry were predictive of a slower drop in ALSFRS-R (p = 0.01) and VC (p < 0.0001), and longer survival (p = 0.02). Higher levels of creatinine at baseline were predictive of a slower drop in ALSFRS-R (p = 0.01) and VC (p < 0.0001), and longer survival (p = 0.01). Finally, higher body mass index (BMI) at baseline was associated with longer survival (p < 0.0001).
Conclusion: The PRO-ACT database is the largest publicly available repository of merged ALS clinical trials data. We report that baseline levels of creatinine and uric acid, as well as baseline BMI, are strong predictors of disease progression and survival.
© 2014 American Academy of Neurology.
Figures

References
-
- Rowland L, Shneider N. Amyotrophic lateral sclerosis. N Engl J Med 2001;344:1688–1700. - PubMed
-
- McGuire V, Longstreth W, Jr, Koepsell T, van Belle G. Incidence of amyotrophic lateral sclerosis in three counties in western Washington state. Neurology 1996;47:571–573. - PubMed
-
- Cudkowicz M, Katz J, Moore D, et al. Toward more efficient clinical trials for amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2010;11:259–265. - PubMed
-
- Molenberghs G, Kenward MG. The Direct Likelihood Method: Missing Data in Clinical Studies. Hoboken, NJ: John Wiley & Sons; 2007:75–92.
-
- Bhattacharjee Y. Pharma firms push for sharing of cancer trial data. Science 2012;338:29. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
