Diagnostic accuracy of pulmonary host inflammatory mediators in the exclusion of ventilator-acquired pneumonia
- PMID: 25298325
- PMCID: PMC4992819
- DOI: 10.1136/thoraxjnl-2014-205766
Diagnostic accuracy of pulmonary host inflammatory mediators in the exclusion of ventilator-acquired pneumonia
Abstract
Background: Excessive use of empirical antibiotics is common in critically ill patients. Rapid biomarker-based exclusion of infection may improve antibiotic stewardship in ventilator-acquired pneumonia (VAP). However, successful validation of the usefulness of potential markers in this setting is exceptionally rare.
Objectives: We sought to validate the capacity for specific host inflammatory mediators to exclude pneumonia in patients with suspected VAP.
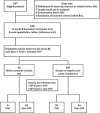
Methods: A prospective, multicentre, validation study of patients with suspected VAP was conducted in 12 intensive care units. VAP was confirmed following bronchoscopy by culture of a potential pathogen in bronchoalveolar lavage fluid (BALF) at >10(4) colony forming units per millilitre (cfu/mL). Interleukin-1 beta (IL-1β), IL-8, matrix metalloproteinase-8 (MMP-8), MMP-9 and human neutrophil elastase (HNE) were quantified in BALF. Diagnostic utility was determined for biomarkers individually and in combination.
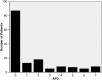
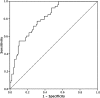
Results: Paired BALF culture and biomarker results were available for 150 patients. 53 patients (35%) had VAP and 97 (65%) patients formed the non-VAP group. All biomarkers were significantly higher in the VAP group (p<0.001). The area under the receiver operator characteristic curve for IL-1β was 0.81; IL-8, 0.74; MMP-8, 0.76; MMP-9, 0.79 and HNE, 0.78. A combination of IL-1β and IL-8, at the optimal cut-point, excluded VAP with a sensitivity of 100%, a specificity of 44.3% and a post-test probability of 0% (95% CI 0% to 9.2%).
Conclusions: Low BALF IL-1β in combination with IL-8 confidently excludes VAP and could form a rapid biomarker-based rule-out test, with the potential to improve antibiotic stewardship.
Keywords: Pneumonia.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
Comment in
-
Biomarkers to exclude the diagnosis of ventilator-associated pneumonia.Thorax. 2015 Jan;70(1):5-6. doi: 10.1136/thoraxjnl-2014-206280. Epub 2014 Oct 28. Thorax. 2015. PMID: 25352532 No abstract available.
References
-
- Vincent JL, Rello J, Marshall J et al. . International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009;302:2323–9. - PubMed
-
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002;165:867–903. - PubMed
-
- Meduri GU, Mauldin GL, Wunderink RG et al. . Causes of fever and pulmonary densities in patients with clinical manifestations of ventilator-associated pneumonia. Chest 1994;106:221–35. - PubMed
-
- Fagon JY, Chastre J, Hance AJ et al. . Detection of nosocomial lung infection in ventilated patients: use of a protected specimen brush and quantitative culture techniques in 147 patients. Am J Respir Crit Care Med 1988;138:110–16. - PubMed
-
- Iregui M, Ward S, Sherman G et al. . Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest 2002;122:262–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
