Biocompatibility and toxicity of poly(vinyl alcohol)/N,O-carboxymethyl chitosan scaffold
- PMID: 25298970
- PMCID: PMC4179949
- DOI: 10.1155/2014/905103
Biocompatibility and toxicity of poly(vinyl alcohol)/N,O-carboxymethyl chitosan scaffold
Abstract
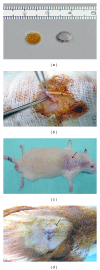
The in vivo biocompatibility and toxicity of PVA/NOCC scaffold were tested by comparing them with those of a biocompatible inert material HAM in a rat model. On Day 5, changes in the blood parameters of the PVA/NOCC-implanted rats were significantly higher than those of the control. The levels of potassium, creatinine, total protein, A/G, hemoglobulin, erythrocytes, WBC, and platelets were not significantly altered in the HAM-implanted rats, when compared with those in the control. On Day 10, an increase in potassium, urea, and GGT levels and a decrease in ALP, platelet, and eosinophil levels were noted in the PVA/NOCC-implanted rats, when compared with control. These changes were almost similar to those noted in the HAM-implanted rats, except for the unaltered potassium and increased neutrophil levels. On Day 15, the total protein, A/G, lymphocyte, monocyte, and eosinophil levels remained unaltered in the PVA/NOCC-implanted rats, whereas urea, A/G, WBC, lymphocyte, and monocyte levels remained unchanged in the HAM-implanted rats. Histology and immunohistochemistry analyses revealed inflammatory infiltration in the PVA/NOCC-implanted rats, but not in the HAM-implanted rats. Although a low toxic tissue response was observed in the PVA/NOCC-implanted rats, further studies are necessary to justify the use of this material in tissue engineering applications.
Figures


References
-
- Arora M, Jaroudi KA, Hamilton CJCM, Dayel F. Controlled comparison of interceed and amniotic membrane graft in the prevention of postoperative adhesions in the rabbit uterine horn model. European Journal of Obstetrics Gynecology & Reproductive Biology. 1994;55(3):179–182. - PubMed
-
- Puppi D, Piras AM, Detta N, et al. Poly(vinyl alcohol)-based electrospun meshes as potential candidate scaffolds in regenerative medicine. Journal of Bioactive and Compatible Polymers. 2011;26(1):20–34.
-
- Pal K, Banthia AK, Majumdar DK. Polyvinyl alcohol-glycine composite membranes: Preparation, characterization, drug release and cytocompatibility studies. Biomedical Materials. 2006;1(2, article 1):49–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

