mRNA Structural constraints on EBNA1 synthesis impact on in vivo antigen presentation and early priming of CD8+ T cells
- PMID: 25299404
- PMCID: PMC4192603
- DOI: 10.1371/journal.ppat.1004423
mRNA Structural constraints on EBNA1 synthesis impact on in vivo antigen presentation and early priming of CD8+ T cells
Abstract
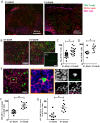
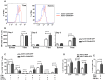
Recent studies have shown that virally encoded mRNA sequences of genome maintenance proteins from herpesviruses contain clusters of unusual structural elements, G-quadruplexes, which modulate viral protein synthesis. Destabilization of these G-quadruplexes can override the inhibitory effect on self-synthesis of these proteins. Here we show that the purine-rich repetitive mRNA sequence of Epstein-Barr virus encoded nuclear antigen 1 (EBNA1) comprising G-quadruplex structures, limits both the presentation of MHC class I-restricted CD8(+) T cell epitopes by CD11c(+) dendritic cells in draining lymph nodes and early priming of antigen-specific CD8(+) T-cells. Destabilization of the G-quadruplex structures through codon-modification significantly enhanced in vivo antigen presentation and activation of virus-specific T cells. Ex vivo imaging of draining lymph nodes by confocal microscopy revealed enhanced antigen-specific T-cell trafficking and APC-CD8(+) T-cell interactions in mice primed with viral vectors encoding a codon-modified EBNA1 protein. More importantly, these antigen-specific T cells displayed enhanced expression of the T-box transcription factor and superior polyfunctionality consistent with the qualitative impact of translation efficiency. These results provide an important insight into how viruses exploit mRNA structure to down regulate synthesis of their viral maintenance proteins and delay priming of antigen-specific T cells, thereby establishing a successful latent infection in vivo. Furthermore, targeting EBNA1 mRNA rather than protein by small molecules or antisense oligonucleotides will enhance EBNA1 synthesis and the early priming of effector T cells, to establish a more rapid immune response and prevent persistent infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Messenger RNA sequence rather than protein sequence determines the level of self-synthesis and antigen presentation of the EBV-encoded antigen, EBNA1.PLoS Pathog. 2012 Dec;8(12):e1003112. doi: 10.1371/journal.ppat.1003112. Epub 2012 Dec 27. PLoS Pathog. 2012. PMID: 23300450 Free PMC article.
-
G-quadruplexes regulate Epstein-Barr virus-encoded nuclear antigen 1 mRNA translation.Nat Chem Biol. 2014 May;10(5):358-64. doi: 10.1038/nchembio.1479. Epub 2014 Mar 16. Nat Chem Biol. 2014. PMID: 24633353 Free PMC article.
-
Translation efficiency of EBNA1 encoded by lymphocryptoviruses influences endogenous presentation of CD8+ T cell epitopes.Eur J Immunol. 2007 Feb;37(2):328-37. doi: 10.1002/eji.200636153. Eur J Immunol. 2007. PMID: 17236233
-
Epstein-Barr virus evasion of CD8(+) and CD4(+) T cell immunity via concerted actions of multiple gene products.Semin Cancer Biol. 2008 Dec;18(6):397-408. doi: 10.1016/j.semcancer.2008.10.008. Epub 2008 Oct 25. Semin Cancer Biol. 2008. PMID: 18977445 Review.
-
Epstein-Barr virus-encoded EBNA1 and ZEBRA: targets for therapeutic strategies against EBV-carrying cancers.J Pathol. 2015 Jan;235(2):334-41. doi: 10.1002/path.4431. J Pathol. 2015. PMID: 25186125 Review.
Cited by
-
G-Quadruplexes in the Viral Genome: Unlocking Targets for Therapeutic Interventions and Antiviral Strategies.Viruses. 2023 Nov 5;15(11):2216. doi: 10.3390/v15112216. Viruses. 2023. PMID: 38005893 Free PMC article. Review.
-
Viral G-quadruplexes: New frontiers in virus pathogenesis and antiviral therapy.Annu Rep Med Chem. 2020;54:101-131. doi: 10.1016/bs.armc.2020.04.001. Epub 2020 May 18. Annu Rep Med Chem. 2020. PMID: 32427223 Free PMC article.
-
G-quadruplexes in pathogens: a common route to virulence control?PLoS Pathog. 2015 Feb 5;11(2):e1004562. doi: 10.1371/journal.ppat.1004562. eCollection 2015 Feb. PLoS Pathog. 2015. PMID: 25654363 Free PMC article. Review.
-
Immune control of oncogenic γ-herpesviruses.Curr Opin Virol. 2015 Oct;14:79-86. doi: 10.1016/j.coviro.2015.08.014. Epub 2015 Sep 13. Curr Opin Virol. 2015. PMID: 26372881 Free PMC article. Review.
-
Unfolding of an RNA G-quadruplex motif in the negative strand genome of porcine reproductive and respiratory syndrome virus by host and viral helicases to promote viral replication.Nucleic Acids Res. 2023 Oct 27;51(19):10752-10767. doi: 10.1093/nar/gkad759. Nucleic Acids Res. 2023. PMID: 37739415 Free PMC article.
References
-
- Yewdell JW (2005) Serendipity strikes twice: the discovery and rediscovery of defective ribosomal products (DRiPS). Cell Mol Biol (Noisy-le-grand) 51: 635–641. - PubMed
-
- Yewdell JW, Anton LC, Bennink JR (1996) Defective ribosomal products (DRiPs): a major source of antigenic peptides for MHC class I molecules? J Immunol 157: 1823–1826. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

