The formation of endoderm-derived taste sensory organs requires a Pax9-dependent expansion of embryonic taste bud progenitor cells
- PMID: 25299669
- PMCID: PMC4191947
- DOI: 10.1371/journal.pgen.1004709
The formation of endoderm-derived taste sensory organs requires a Pax9-dependent expansion of embryonic taste bud progenitor cells
Abstract
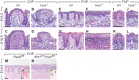
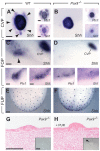
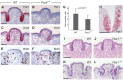
In mammals, taste buds develop in different regions of the oral cavity. Small epithelial protrusions form fungiform papillae on the ectoderm-derived dorsum of the tongue and contain one or few taste buds, while taste buds in the soft palate develop without distinct papilla structures. In contrast, the endoderm-derived circumvallate and foliate papillae located at the back of the tongue contain a large number of taste buds. These taste buds cluster in deep epithelial trenches, which are generated by intercalating a period of epithelial growth between initial placode formation and conversion of epithelial cells into sensory cells. How epithelial trench formation is genetically regulated during development is largely unknown. Here we show that Pax9 acts upstream of Pax1 and Sox9 in the expanding taste progenitor field of the mouse circumvallate papilla. While a reduced number of taste buds develop in a growth-retarded circumvallate papilla of Pax1 mutant mice, its development arrests completely in Pax9-deficient mice. In addition, the Pax9 mutant circumvallate papilla trenches lack expression of K8 and Prox1 in the taste bud progenitor cells, and gradually differentiate into an epidermal-like epithelium. We also demonstrate that taste placodes of the soft palate develop through a Pax9-dependent induction. Unexpectedly, Pax9 is dispensable for patterning, morphogenesis and maintenance of taste buds that develop in ectoderm-derived fungiform papillae. Collectively, our data reveal an endoderm-specific developmental program for the formation of taste buds and their associated papilla structures. In this pathway, Pax9 is essential to generate a pool of taste bud progenitors and to maintain their competence towards prosensory cell fate induction.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Expression of the basal cell markers of taste buds in the anterior tongue and soft palate of the mouse embryo.J Comp Neurol. 2008 Jul 10;509(2):211-24. doi: 10.1002/cne.21738. J Comp Neurol. 2008. PMID: 18465790
-
Multiple Shh signaling centers participate in fungiform papilla and taste bud formation and maintenance.Dev Biol. 2013 Oct 1;382(1):82-97. doi: 10.1016/j.ydbio.2013.07.022. Epub 2013 Aug 2. Dev Biol. 2013. PMID: 23916850 Free PMC article.
-
Temporal and spatial patterns of tenascin and laminin immunoreactivity suggest roles for extracellular matrix in development of gustatory papillae and taste buds.J Comp Neurol. 1996 Jan 15;364(3):535-555. doi: 10.1002/(SICI)1096-9861(19960115)364:3<535::AID-CNE11>3.0.CO;2-O. J Comp Neurol. 1996. PMID: 8820882
-
Development of fungiform papillae: patterned lingual gustatory organs.Arch Histol Cytol. 2006 Dec;69(4):199-208. doi: 10.1679/aohc.69.199. Arch Histol Cytol. 2006. PMID: 17287575 Review.
-
Development of ectodermal and endodermal taste buds.Dev Biol. 2025 Feb;518:20-27. doi: 10.1016/j.ydbio.2024.10.005. Epub 2024 Oct 30. Dev Biol. 2025. PMID: 39486632 Review.
Cited by
-
Altered salt taste response and increased tongue epithelium Scnna1 expression in adult Engrailed-2 null mice.Physiol Behav. 2018 Oct 1;194:410-419. doi: 10.1016/j.physbeh.2018.06.030. Epub 2018 Jun 25. Physiol Behav. 2018. PMID: 29953887 Free PMC article.
-
Action of Actomyosin Contraction With Shh Modulation Drive Epithelial Folding in the Circumvallate Papilla.Front Physiol. 2020 Jul 31;11:936. doi: 10.3389/fphys.2020.00936. eCollection 2020. Front Physiol. 2020. PMID: 32848868 Free PMC article.
-
FGF10 Is Required for Circumvallate Papilla Morphogenesis by Maintaining Lgr5 Activity.Front Physiol. 2018 Aug 28;9:1192. doi: 10.3389/fphys.2018.01192. eCollection 2018. Front Physiol. 2018. PMID: 30233392 Free PMC article.
-
PAX9 in Cancer Development.Int J Mol Sci. 2022 May 17;23(10):5589. doi: 10.3390/ijms23105589. Int J Mol Sci. 2022. PMID: 35628401 Free PMC article. Review.
-
An overview of PAX1: Expression, function and regulation in development and diseases.Front Cell Dev Biol. 2022 Oct 28;10:1051102. doi: 10.3389/fcell.2022.1051102. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36393845 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

