Light-scattering detection below the level of single fluorescent molecules for high-resolution characterization of functional nanoparticles
- PMID: 25300001
- PMCID: PMC4212780
- DOI: 10.1021/nn505162u
Light-scattering detection below the level of single fluorescent molecules for high-resolution characterization of functional nanoparticles
Abstract
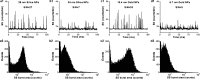
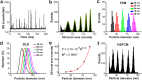
Ultrasensitive detection and characterization of single nanoparticles (<100 nm) is important in nanotechnology and life sciences. Direct measurement of the elastically scattered light from individual nanoparticles represents the simplest and the most direct method for particle detection. However, the sixth-power dependence of scattering intensity on particle size renders very small particles indistinguishable from the background. Adopting strategies for single-molecule fluorescence detection in a sheathed flow, here we report the development of high sensitivity flow cytometry (HSFCM) that achieves real-time light-scattering detection of single silica and gold nanoparticles as small as 24 and 7 nm in diameter, respectively. This unprecedented sensitivity enables high-resolution sizing of single nanoparticles directly based on their scattered intensity. With a resolution comparable to that of TEM and the ease and speed of flow cytometric analysis, HSFCM is particularly suitable for nanoparticle size distribution analysis of polydisperse/heterogeneous/mixed samples. Through concurrent fluorescence detection, simultaneous insights into the size and payload variations of engineered nanoparticles are demonstrated with two forms of clinical nanomedicine. By offering quantitative multiparameter analysis of single nanoparticles in liquid suspensions at a throughput of up to 10 000 particles per minute, HSFCM represents a major advance both in light-scattering detection technology and in nanoparticle characterization.
Keywords: flow cytometry; light scattering; nanomedicine; nanoparticle characterization; single-molecule detection; single-nanoparticle detection; size distribution.
Figures



References
-
- Abeylath S. C.; Ganta S.; Iyer A. K.; Amiji M. Combinatorial-Designed Multifunctional Polymeric Nanosystems for Tumor-Targeted Therapeutic Delivery. Acc. Chem. Res. 2011, 44, 1009–1017. - PubMed
-
- Bourzac K. Nanotechnology: Carrying Drugs. Nature 2012, 491, S58–S60. - PubMed
-
- Hubbell J. A.; Langer R. Translating Materials Design to the Clinic. Nat. Mater. 2013, 12, 963–966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

