Celastrol, an oral heat shock activator, ameliorates multiple animal disease models of cell death
- PMID: 25300203
- PMCID: PMC4255245
- DOI: 10.1007/s12192-014-0536-1
Celastrol, an oral heat shock activator, ameliorates multiple animal disease models of cell death
Abstract
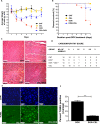
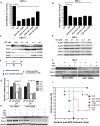
Protein homeostatic regulators have been shown to ameliorate single, loss-of-function protein diseases but not to treat broader animal disease models that may involve cell death. Diseases often trigger protein homeostatic instability that disrupts the delicate balance of normal cellular viability. Furthermore, protein homeostatic regulators have been delivered invasively and not with simple oral administration. Here, we report the potent homeostatic abilities of celastrol to promote cell survival, decrease inflammation, and maintain cellular homeostasis in three different disease models of apoptosis and inflammation involving hepatocytes and cardiomyocytes. We show that celastrol significantly recovers the left ventricular function and myocardial remodeling following models of acute myocardial infarction and doxorubicin-induced cardiomyopathy by diminishing infarct size, apoptosis, and inflammation. Celastrol prevents acute liver dysfunction and promotes hepatocyte survival after toxic doses of thioacetamide. Finally, we show that heat shock response (HSR) is necessary and sufficient for the recovery abilities of celastrol. Our observations may have dramatic clinical implications to ameliorate entire disease processes even after cellular injury initiation by using an orally delivered HSR activator.
Figures




Similar articles
-
Celastrol protects ischaemic myocardium through a heat shock response with up-regulation of haeme oxygenase-1.Br J Pharmacol. 2014 Dec;171(23):5265-79. doi: 10.1111/bph.12838. Br J Pharmacol. 2014. PMID: 25041185 Free PMC article.
-
Celastrol supports survival of retinal ganglion cells injured by optic nerve crush.Brain Res. 2015 Jun 3;1609:21-30. doi: 10.1016/j.brainres.2015.03.032. Epub 2015 Mar 24. Brain Res. 2015. PMID: 25813825 Free PMC article.
-
Induction of heat shock proteins in differentiated human neuronal cells following co-application of celastrol and arimoclomol.Cell Stress Chaperones. 2016 Sep;21(5):837-48. doi: 10.1007/s12192-016-0708-2. Epub 2016 Jun 8. Cell Stress Chaperones. 2016. PMID: 27273088 Free PMC article.
-
Activation of heat shock and antioxidant responses by the natural product celastrol: transcriptional signatures of a thiol-targeted molecule.Mol Biol Cell. 2008 Mar;19(3):1104-12. doi: 10.1091/mbc.e07-10-1004. Epub 2008 Jan 16. Mol Biol Cell. 2008. PMID: 18199679 Free PMC article.
-
Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases.Nat Rev Drug Discov. 2011 Dec 1;10(12):930-44. doi: 10.1038/nrd3453. Nat Rev Drug Discov. 2011. PMID: 22129991 Free PMC article. Review.
Cited by
-
Inhibition of mitochondrial respiration has fundamentally different effects on proliferation, cell survival and stress response in immature versus differentiated cardiomyocyte cell lines.Front Cell Dev Biol. 2022 Sep 23;10:1011639. doi: 10.3389/fcell.2022.1011639. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36211452 Free PMC article.
-
Loss of heat shock factor 1 promotes hepatic stellate cell activation and drives liver fibrosis.Hepatol Commun. 2022 Oct;6(10):2781-2797. doi: 10.1002/hep4.2058. Epub 2022 Aug 9. Hepatol Commun. 2022. PMID: 35945902 Free PMC article.
-
Protein Quality Control and the Amyotrophic Lateral Sclerosis/Frontotemporal Dementia Continuum.Front Mol Neurosci. 2017 May 10;10:119. doi: 10.3389/fnmol.2017.00119. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28539871 Free PMC article.
-
SHYCD induces APE1/Ref-1 subcellular localization to regulate the p53-apoptosis signaling pathway in the prevention and treatment of acute on chronic liver failure.Oncotarget. 2017 Aug 4;8(49):84782-84797. doi: 10.18632/oncotarget.19891. eCollection 2017 Oct 17. Oncotarget. 2017. PMID: 29156683 Free PMC article.
-
The Role of HSP90 Inhibitors in the Treatment of Cardiovascular Diseases.Cells. 2022 Oct 31;11(21):3444. doi: 10.3390/cells11213444. Cells. 2022. PMID: 36359837 Free PMC article. Review.
References
-
- Bagatell R, Paine-Murrieta GD, Taylor CW, Pulcini EJ, Akinaga S, Benjamin IJ, Whitesell L. Induction of a heat shock factor 1-dependent stress response alters the cytotoxic activity of hsp90-binding agents. Clin Cancer Res : Off J Am Assoc Cancer Res. 2000;6:3312–3318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

