β-catenin links hepatic metabolic zonation with lipid metabolism and diet-induced obesity in mice
- PMID: 25300578
- PMCID: PMC4258504
- DOI: 10.1016/j.ajpath.2014.08.022
β-catenin links hepatic metabolic zonation with lipid metabolism and diet-induced obesity in mice
Abstract
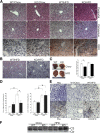
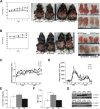
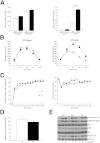
β-catenin regulates the establishment of hepatic metabolic zonation. To elucidate the functional significance of liver metabolic zonation in the chronically overfed state in vivo, we fed a high-fat diet (HFD) to hepatocyte-specific β-catenin transgenic (TG) and knockout (KO) mice. Chow-fed TG and KO mice had normal liver histologic findings and body weight. However, HFD-fed TG mice developed prominent perivenous steatosis with periportal sparing. In contrast, HFD-fed KO mice had increased lobular inflammation and hepatocyte apoptosis. HFD-fed TG mice rapidly developed diet-induced obesity and systemic insulin resistance, but KO mice were resistant to diet-induced obesity. However, β-catenin did not directly affect hepatic insulin signaling, suggesting that the metabolic effects of β-catenin occurred via a parallel pathway. Hepatic expression of key glycolytic and lipogenic genes was higher in HFD-fed TG and lower in KO mice compared with wild-type mice. KO mice also exhibited defective hepatic fatty acid oxidation and fasting ketogenesis. Hepatic levels of hypoxia inducible factor-1α, an oxygen-sensitive transcriptional regulator of glycolysis and a known β-catenin binding partner, were higher in HFD-fed TG and lower in KO mice. KO mice had attenuated perivenous hypoxia, suggesting disruption of the normal sinusoidal oxygen gradient, a major determinant of liver carbohydrate and liver metabolism. Canonical Wnt signaling in hepatocytes is essential for the development of diet-induced fatty liver and obesity.
Copyright © 2014 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Owen O.E., Reichard G.A., Jr., Patel M.S., Boden G. Energy metabolism in feasting and fasting. Adv Exp Med Biol. 1979;111:169–188. - PubMed
-
- Jungermann K. Metabolic zonation of liver parenchyma. Semin Liver Dis. 1988;8:329–341. - PubMed
-
- Jungermann K., Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989;69:708–764. - PubMed
-
- Benhamouche S., Decaens T., Godard C., Chambrey R., Rickman D.S., Moinard C., Vasseur-Cognet M., Kuo C.J., Kahn A., Perret C., Colnot S. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell. 2006;10:759–770. - PubMed
-
- Behari J., Yeh T.H., Krauland L., Otruba W., Cieply B., Hauth B., Apte U., Wu T., Evans R., Monga S.P. Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis. Am J Pathol. 2010;176:744–753. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

