Regulation and biological function of a flagellar glucose transporter in Leishmania mexicana: a potential glucose sensor
- PMID: 25300620
- PMCID: PMC4285543
- DOI: 10.1096/fj.14-251991
Regulation and biological function of a flagellar glucose transporter in Leishmania mexicana: a potential glucose sensor
Abstract
In Leishmania mexicana parasites, a unique glucose transporter, LmxGT1, is selectively targeted to the flagellar membrane, suggesting a possible sensory role that is often associated with ciliary membrane proteins. Expression of LmxGT1 is down-regulated ∼20-fold by increasing cell density but is up-regulated ∼50-fold by depleting glucose from the medium, and the permease is strongly down-regulated when flagellated insect-stage promastigotes invade mammalian macrophages and transform into intracellular amastigotes. Regulation of LmxGT1 expression by glucose and during the lifecycle operates at the level of protein stability. Significantly, a ∆lmxgt1 null mutant, grown in abundant glucose, undergoes catastrophic loss of viability when parasites deplete glucose from the medium, a property not exhibited by wild-type or add-back lines. These results suggest that LmxGT1 may function as a glucose sensor that allows parasites to enter the stationary phase when they deplete glucose and that in the absence of this sensor, parasites do not maintain viability when they run out of glucose. However, alternate roles for LmxGT1 in monitoring glucose availability are considered. The absence of known sensory receptors with defined ligands and biologic functions in Leishmania and related kinetoplastid parasites underscores the potential significance of these observations.
Keywords: Leishmania parasites; TaV2A peptide; environmental sensing; protein expression; transceptor.
© FASEB.
Figures
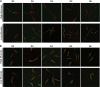
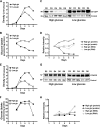


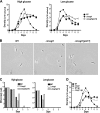

Comment in
-
Transporters, channels and receptors in flagella.Channels (Austin). 2014;8(6):477-8. doi: 10.4161/19336950.2014.985481. Channels (Austin). 2014. PMID: 25485659 Free PMC article. No abstract available.
References
-
- Rodrigues J. C., Godinho J. L., and de Souza W. (2014) Biology of human pathogenic trypanosomatids: epidemiology, lifecycle and ultrastructure. Subcell. Biochem. 74, 1–42 - PubMed
-
- Gluenz E., Ginger M. L., and McKean P. G. (2010) Flagellum assembly and function during the Leishmania life cycle. Curr. Opin. Microbiol. 13, 473–479 - PubMed
-
- Naderer T., and McConville M. J. (2008) The Leishmania-macrophage interaction: a metabolic perspective. Cell. Microbiol. 10, 301–308 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

