Neuroprotection requires the functions of the RNA-binding protein HuR
- PMID: 25301069
- PMCID: PMC4392069
- DOI: 10.1038/cdd.2014.158
Neuroprotection requires the functions of the RNA-binding protein HuR
Abstract
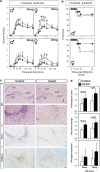
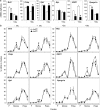
Alterations in the functions of neuronal RNA-binding proteins (RBPs) can contribute to neurodegenerative diseases. However, neurons also express a set of widely distributed RBPs that may have developed specialized functions. Here, we show that the ubiquitous member of the otherwise neuronal Elavl/Hu family of RNA-binding proteins, Elavl1/HuR, has a neuroprotective role. Mice engineered to lack exclusively HuR in the hippocampal neurons of the central nervous system (CNS), maintain physiologic levels of neuronal Elavls and develop a partially diminished seizure response following strong glutamatergic excitation; however, they display an exacerbated neurodegenerative response subsequent to the initial excitotoxic event. This response was phenocopied in hippocampal cells devoid of ionotropic glutamate receptors in which the loss of HuR results in enhanced mitochondrial dysfunction, oxidative damage and programmed necrosis solely after glutamate challenge. The molecular dissection of HuR and nElavl mRNA targets revealed the existence of a HuR-restricted posttranscriptional regulon that failed in HuR-deficient neurons and is involved in cellular energetics and oxidation defense. Thus, HuR acts as a specialized controller of oxidative metabolism in neurons to confer protection from neurodegeneration.
Figures





Similar articles
-
Interleukin 1β Regulation of the System xc- Substrate-specific Subunit, xCT, in Primary Mouse Astrocytes Involves the RNA-binding Protein HuR.J Biol Chem. 2016 Jan 22;291(4):1643-1651. doi: 10.1074/jbc.M115.697821. Epub 2015 Nov 24. J Biol Chem. 2016. PMID: 26601945 Free PMC article.
-
RNA-Binding Protein HuR Regulates Paneth Cell Function by Altering Membrane Localization of TLR2 via Post-transcriptional Control of CNPY3.Gastroenterology. 2019 Sep;157(3):731-743. doi: 10.1053/j.gastro.2019.05.010. Epub 2019 May 17. Gastroenterology. 2019. PMID: 31103627 Free PMC article.
-
Detrimental ELAVL-1/HuR-dependent GSK3β mRNA stabilization impairs resolution in acute respiratory distress syndrome.PLoS One. 2017 Feb 14;12(2):e0172116. doi: 10.1371/journal.pone.0172116. eCollection 2017. PLoS One. 2017. PMID: 28196122 Free PMC article.
-
Targeting the RNA-Binding Protein HuR as Potential Thera-Peutic Approach for Neurological Disorders: Focus on Amyo-Trophic Lateral Sclerosis (ALS), Spinal Muscle Atrophy (SMA) and Multiple Sclerosis.Int J Mol Sci. 2021 Sep 27;22(19):10394. doi: 10.3390/ijms221910394. Int J Mol Sci. 2021. PMID: 34638733 Free PMC article. Review.
-
Human antigen R: Exploring its inflammatory response impact and significance in cardiometabolic disorders.J Cell Physiol. 2024 May;239(5):e31229. doi: 10.1002/jcp.31229. Epub 2024 Mar 1. J Cell Physiol. 2024. PMID: 38426269 Review.
Cited by
-
Stress response regulation of mRNA translation: Implications for antioxidant enzyme expression in cancer.Proc Natl Acad Sci U S A. 2024 Nov 12;121(46):e2317846121. doi: 10.1073/pnas.2317846121. Epub 2024 Nov 4. Proc Natl Acad Sci U S A. 2024. PMID: 39495917 Free PMC article. Review.
-
Two Thalamic Regions Screened Using Laser Capture Microdissection with Whole Human Genome Microarray in Schizophrenia Postmortem Samples.Schizophr Res Treatment. 2020 May 31;2020:5176834. doi: 10.1155/2020/5176834. eCollection 2020. Schizophr Res Treatment. 2020. PMID: 32566292 Free PMC article.
-
HuR controls apoptosis and activation response without effects on cytokine 3' UTRs.RNA Biol. 2019 May;16(5):686-695. doi: 10.1080/15476286.2019.1582954. Epub 2019 Mar 4. RNA Biol. 2019. PMID: 30777501 Free PMC article.
-
Cell-Penetrating Peptide TAT-HuR-HNS3 Suppresses Proinflammatory Gene Expression via Competitively Blocking Interaction of HuR with Its Partners.J Immunol. 2022 May 15;208(10):2376-2389. doi: 10.4049/jimmunol.2200002. Epub 2022 Apr 20. J Immunol. 2022. PMID: 35444028 Free PMC article.
-
Tau Modulates mRNA Transcription, Alternative Polyadenylation Profiles of hnRNPs, Chromatin Remodeling and Spliceosome Complexes.Front Mol Neurosci. 2021 Dec 3;14:742790. doi: 10.3389/fnmol.2021.742790. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34924950 Free PMC article.
References
-
- Wang Y, Qin ZH. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis. 2010;15:1382–1402. - PubMed
-
- Danbolt N. Glutamate uptake. Progr Neurobiol. 2001;65:1–105. - PubMed
-
- Michaelis E. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Progr Neurobiol. 1998;54:369–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

