Bryostatin-1 Restores Blood Brain Barrier Integrity following Blast-Induced Traumatic Brain Injury
- PMID: 25301233
- PMCID: PMC5000781
- DOI: 10.1007/s12035-014-8902-7
Bryostatin-1 Restores Blood Brain Barrier Integrity following Blast-Induced Traumatic Brain Injury
Abstract
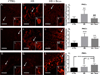
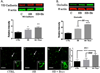
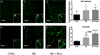
Recent wars in Iraq and Afghanistan have accounted for an estimated 270,000 blast exposures among military personnel. Blast traumatic brain injury (TBI) is the 'signature injury' of modern warfare. Blood brain barrier (BBB) disruption following blast TBI can lead to long-term and diffuse neuroinflammation. In this study, we investigate for the first time the role of bryostatin-1, a specific protein kinase C (PKC) modulator, in ameliorating BBB breakdown. Thirty seven Sprague-Dawley rats were used for this study. We utilized a clinically relevant and validated blast model to expose animals to moderate blast exposure. Groups included: control, single blast exposure, and single blast exposure + bryostatin-1. Bryostatin-1 was administered i.p. 2.5 mg/kg after blast exposure. Evan's blue, immunohistochemistry, and western blot analysis were performed to assess injury. Evan's blue binds to albumin and is a marker for BBB disruption. The single blast exposure caused an increase in permeability compared to control (t = 4.808, p < 0.05), and a reduction back toward control levels when bryostatin-1 was administered (t = 5.113, p < 0.01). Three important PKC isozymes, PKCα, PKCδ, and PKCε, were co-localized primarily with endothelial cells but not astrocytes. Bryostatin-1 administration reduced toxic PKCα levels back toward control levels (t = 4.559, p < 0.01) and increased the neuroprotective isozyme PKCε (t = 6.102, p < 0.01). Bryostatin-1 caused a significant increase in the tight junction proteins VE-cadherin, ZO-1, and occludin through modulation of PKC activity. Bryostatin-1 ultimately decreased BBB breakdown potentially due to modulation of PKC isozymes. Future work will examine the role of bryostatin-1 in preventing chronic neurodegeneration following repetitive neurotrauma.
Keywords: Blood brain barrier; Bryostatin-1; Protein kinase C; Tight junction proteins.
Conflict of interest statement
The authors claim to have no conflicts of interest.
Figures







References
-
- Kou Z, Vandevord PJ. Traumatic white matter injury and glial activation: from basic science to clinics. Glia. 2014 - PubMed
-
- Hue CD, Cao S, Dale Bass CR, Meaney DF, Morrison B., 3rd Repeated primary blast injury causes delayed recovery, but not additive disruption, in an in vitro blood–brain barrier model. J Neurotrauma. 2014;31(10):951–960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

