Chronic spinal cord stimulation modifies intrinsic cardiac synaptic efficacy in the suppression of atrial fibrillation
- PMID: 25301713
- PMCID: PMC4607028
- DOI: 10.1016/j.autneu.2014.09.017
Chronic spinal cord stimulation modifies intrinsic cardiac synaptic efficacy in the suppression of atrial fibrillation
Abstract
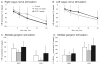
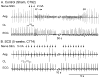
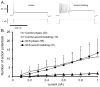
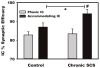
We sought to determine whether spinal cord stimulation (SCS) therapy, when applied chronically to canines, imparts long-lasting cardio-protective effects on neurogenic atrial tachyarrhythmia induction and, if so, whether its effects can be attributable to i) changes in intrinsic cardiac (IC) neuronal transmembrane properties vs ii) modification of their interneuronal stochastic interactivity that initiates such pathology. Data derived from canines subjected to long-term SCS [(group 1: studied after 3-4 weeks SCS; n = 5) (group 2: studied after 5 weeks SCS; n = 11)] were compared to data derived from 10 control animals (including 4 sham SCS electrode implantations). During terminal studies conducted under anesthesia, chronotropic and inotropic responses to vagal nerve or stellate ganglion stimulation were similar in all 3 groups. Chronic SCS suppressed atrial tachyarrhythmia induction evoked by mediastinal nerve stimulation. When induced, arrhythmia durations were shortened (controls: median of 27 s; SCS 3-4 weeks: median of 16s; SCS 5 weeks: median of 7s). Phasic and accommodating right atrial neuronal somata displayed similar passive and active membrane properties in vitro, whether derived from sham or either chronic SCS group. Synaptic efficacy was differentially enhanced in accommodating (not phasic) IC neurons by chronic SCS. Taken together these data indicate that chronic SCS therapy modifies IC neuronal stochastic inter-connectivity in atrial fibrillation suppression by altering synaptic function without directly targeting the transmembrane properties of individual IC neuronal somata.
Keywords: Atrial tachyarrhythmia; Heart; Intrinsic cardiac neurons; Neuromodulation; Spinal cord stimulation.
Copyright © 2014 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures

References
-
- Ajijola OA, Yagishita D, Patel KJ, Vaseghi M, Zhou W, Yamakawa K, So E, Lux RL, Mahajan A, Shivkumar K. Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: neural remodeling in a spatial context. Am J Physiol Heart Circ Physiol. 2013;305:H1031–H1040. - PMC - PubMed
-
- Ardell JL. Intrathoracic neuronal regulation of cardiac function. In: Armour JA, Ardell JL, editors. Basic and Clinical Neurocardiology. Oxford University Press; New York: 2004. pp. 118–152.
-
- Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol. 2008;93(2):165–176. - PubMed
-
- Armour JA, Kember G. Cardiac Sensory Neurons. In: Armour JA, Ardell JL, editors. Basic and Clinical Neurocardiology. Oxford Univ. Press; NY: 2004. pp. 79–117.
-
- Armour JA, Linderoth B, Arora RC, DeJongste MJL, Ardell JL, Kingma JG, Hill M, Foreman RD. Long-term modulation of the intrinsic cardiac nervous system by spinal cord neurons in normal and ischemic hearts. Autonomic Neuroscience: Basic and Clinical. 2002;95:71–79. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

