The endoplasmic reticulum-based acetyltransferases, ATase1 and ATase2, associate with the oligosaccharyltransferase to acetylate correctly folded polypeptides
- PMID: 25301944
- PMCID: PMC4231681
- DOI: 10.1074/jbc.M114.585547
The endoplasmic reticulum-based acetyltransferases, ATase1 and ATase2, associate with the oligosaccharyltransferase to acetylate correctly folded polypeptides
Abstract
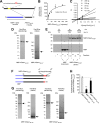
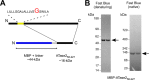
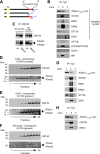
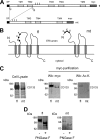
The endoplasmic reticulum (ER) has two membrane-bound acetyltransferases responsible for the endoluminal N(ϵ)-lysine acetylation of ER-transiting and -resident proteins. Mutations that impair the ER-based acetylation machinery are associated with developmental defects and a familial form of spastic paraplegia. Deficient ER acetylation in the mouse leads to defects of the immune and nervous system. Here, we report that both ATase1 and ATase2 form homo- and heterodimers and associate with members of the oligosaccharyltransferase (OST) complex. In contrast to the OST, the ATases only modify correctly folded polypetides. Collectively, our studies suggest that one of the functions of the ATases is to work in concert with the OST and "select" correctly folded from unfolded/misfolded transiting polypeptides.
Keywords: Acetyl Coenzyme A (acetyl-CoA); Acetyltransferase; Endoplasmic Reticulum (ER); Oligosaccharyltransferase; Post-translational Modification (PTM).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., Mann M. (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

