Pharmacokinetics and tolerability of the new second-generation nonnucleoside reverse- transcriptase inhibitor KM-023 in healthy subjects
- PMID: 25302016
- PMCID: PMC4189701
- DOI: 10.2147/DDDT.S65596
Pharmacokinetics and tolerability of the new second-generation nonnucleoside reverse- transcriptase inhibitor KM-023 in healthy subjects
Abstract
Background: KM-023 is a new second-generation nonnucleoside reverse-transcriptase inhibitor that is under development for the treatment of human immunodeficiency virus (HIV) type 1 infection.
Objective: This study determined KM-023 tolerability and pharmacokinetic characteristics in healthy subjects.
Materials and methods: A randomized, double-blinded, placebo-controlled, dose-escalation study was conducted in 80 healthy South Korean male volunteers. The subjects were allocated to single- or multiple-dose (once daily for 7 days) groups that received 75, 150, 300, or 600 mg drug or placebo in a 4:1 ratio. Safety and pharmacokinetic assessments were performed during the study. Plasma and urine concentrations were quantified using liquid chromatography-tandem mass spectrometry.
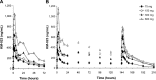
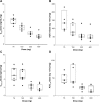
Results: The average maximum concentration (Cmax) and area under the concentration-time curve from time 0 to infinity (AUC∞) values of KM-023 for the 75-600 mg doses in the single-dose study ranged from 440.2 ng/mL to 1,245.4 ng/mL and 11,142.4 ng · h/mL to 33,705.6 ng · h/mL, respectively. Values of the mean Cmax at a steady state and AUC within the dosing interval ranged from 385.1 ng/mL to 1,096.7 ng/mL and 3,698.9 ng · h/mL to 10,232.6 ng · h/mL, respectively, following 75-600 mg doses in the multiple-dose study. Dose proportionality was not observed for KM-023. KM-023 showed a 0.6-fold accumulation after multiple doses in the 600 mg dose group. The mean half-life values ranged between 20.7 and 31.2 hours. KM-023 was generally well tolerated without serious adverse events.
Conclusion: KM-023 demonstrated dose- and time-dependent nonlinear pharmacokinetic characteristics after single or multiple doses over a dose range (75-600 mg) in healthy subjects. KM-023 showed favorable tolerability in this study. This Phase I clinical trial information can be used to design further clinical studies appropriately to evaluate KM-023 in patients with HIV-1 infection.
Keywords: HIV-1; KM-023; healthy subjects; nonnucleoside reverse-transcriptase inhibitor; pharmacokinetics; tolerability.
Figures
References
-
- Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society – USA panel. JAMA. 2012;308(4):387–402. - PubMed
-
- Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. - PubMed
-
- Pauwels R. New non-nucleoside reverse transcriptase inhibitors (NNRTIs) in development for the treatment of HIV infections. Curr Opin Pharmacol. 2004;4(5):437–446. - PubMed
-
- Arastéh K, Rieger A, Yeni P, et al. Short-term randomized proof-of-principle trial of TMC278 in patients with HIV type-1 who have previously failed antiretroviral therapy. Antivir Ther. 2009;14(5):713–722. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

