An optimized probucol microencapsulated formulation integrating a secondary bile acid (deoxycholic acid) as a permeation enhancer
- PMID: 25302020
- PMCID: PMC4189710
- DOI: 10.2147/DDDT.S68247
An optimized probucol microencapsulated formulation integrating a secondary bile acid (deoxycholic acid) as a permeation enhancer
Abstract
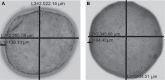
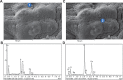
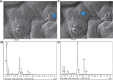
The authors have previously designed, developed, and characterized a novel microencapsulated formulation as a platform for the targeted delivery of therapeutics in an animal model of type 2 diabetes, using the drug probucol (PB). The aim of this study was to optimize PB microcapsules by incorporating the bile acid deoxycholic acid (DCA), which has good permeation-enhancing properties, and to examine its effect on microcapsules' morphology, rheology, structural and surface characteristics, and excipients' chemical and thermal compatibilities. Microencapsulation was carried out using a BÜCHI-based microencapsulating system established in the authors' laboratory. Using the polymer sodium alginate (SA), two microencapsulated formulations were prepared: PB-SA (control) and PB-DCA-SA (test) at a constant ratio (1:30 and 1:3:30, respectively). Complete characterization of the microcapsules was carried out. The incorporation of DCA resulted in better structural and surface characteristics, uniform morphology, and stable chemical and thermal profiles, while size and rheological parameters remained similar to control. In addition, PB-DCA-SA microcapsules showed good excipients' compatibilities, which were supported by data from differential scanning calorimetry, Fourier transform infrared spectroscopy, scanning electron microscopy, and energy dispersive X-ray studies, suggesting microcapsule stability. Hence, PB-DCA-SA microcapsules have good rheological and compatibility characteristics and may be suitable for the oral delivery of PB in type 2 diabetes.
Keywords: BÜCHI B390; anti-inflammatory; antioxidant; artificial cell microencapsulation; bile acids; diabetes; probucol.
Figures




Similar articles
-
The effect of a tertiary bile acid, taurocholic acid, on the morphology and physical characteristics of microencapsulated probucol: potential applications in diabetes: a characterization study.Drug Deliv Transl Res. 2015 Oct;5(5):511-22. doi: 10.1007/s13346-015-0248-9. Drug Deliv Transl Res. 2015. PMID: 26242686
-
Novel chenodeoxycholic acid-sodium alginate matrix in the microencapsulation of the potential antidiabetic drug, probucol. An in vitro study.J Microencapsul. 2015;32(6):589-97. doi: 10.3109/02652048.2015.1065922. Epub 2015 Jul 20. J Microencapsul. 2015. PMID: 26190214
-
Microencapsulation as a novel delivery method for the potential antidiabetic drug, Probucol.Drug Des Devel Ther. 2014 Sep 9;8:1221-30. doi: 10.2147/DDDT.S67349. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 25246766 Free PMC article.
-
Multicompartmental, multilayered probucol microcapsules for diabetes mellitus: Formulation characterization and effects on production of insulin and inflammation in a pancreatic β-cell line.Artif Cells Nanomed Biotechnol. 2016 Nov;44(7):1642-53. doi: 10.3109/21691401.2015.1069299. Epub 2015 Sep 17. Artif Cells Nanomed Biotechnol. 2016. PMID: 26377035
-
Pharmaceutical formulation and polymer chemistry for cell encapsulation applied to the creation of a lab-on-a-chip bio-microsystem.Ther Deliv. 2022 Jan;13(1):51-65. doi: 10.4155/tde-2021-0067. Epub 2021 Nov 25. Ther Deliv. 2022. PMID: 34821516 Review.
Cited by
-
The effect of a tertiary bile acid, taurocholic acid, on the morphology and physical characteristics of microencapsulated probucol: potential applications in diabetes: a characterization study.Drug Deliv Transl Res. 2015 Oct;5(5):511-22. doi: 10.1007/s13346-015-0248-9. Drug Deliv Transl Res. 2015. PMID: 26242686
-
Pharmacological and Biological Study of Microencapsulated Probucol-Secondary Bile Acid in a Diseased Mouse Model.Pharmaceutics. 2021 Aug 8;13(8):1223. doi: 10.3390/pharmaceutics13081223. Pharmaceutics. 2021. PMID: 34452184 Free PMC article.
-
Micro-Nano formulation of bile-gut delivery: rheological, stability and cell survival, basal and maximum respiration studies.Sci Rep. 2020 May 7;10(1):7715. doi: 10.1038/s41598-020-64355-z. Sci Rep. 2020. PMID: 32382021 Free PMC article.
-
Chenodeoxycholic Acid Pharmacology in Biotechnology and Transplantable Pharmaceutical Applications for Tissue Delivery: An Acute Preclinical Study.Cells. 2021 Sep 16;10(9):2437. doi: 10.3390/cells10092437. Cells. 2021. PMID: 34572086 Free PMC article.
-
Pharmacological and Advanced Cell Respiration Effects, Enhanced by Toxic Human-Bile Nano-Pharmaceuticals of Probucol Cell-Targeting Formulations.Pharmaceutics. 2020 Jul 29;12(8):708. doi: 10.3390/pharmaceutics12080708. Pharmaceutics. 2020. PMID: 32751051 Free PMC article.
References
-
- Barnett R. Historical keyword: diabetes. Lancet. 2010;375(9710):191. - PubMed
-
- Torpy JM, Lynm C, Glass RM. JAMA patient page: diabetes. JAMA. 2009;301(15):1620. - PubMed
-
- Barbeau WE, Bassaganya-Riera J, Hontecillas R. Putting the pieces of the puzzle together – a series of hypotheses on the etiology and pathogenesis of type 1 diabetes. Med Hypotheses. 2007;68(3):607–619. - PubMed
-
- Moore PA, Zgibor JC, Dasanayake AP. Diabetes: a growing epidemic of all ages. J Am Dent Assoc. 2003;134(Spec No):11S–15S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

