Enhancement of osseointegration of polyethylene terephthalate artificial ligament by coating of silk fibroin and depositing of hydroxyapatite
- PMID: 25302023
- PMCID: PMC4189703
- DOI: 10.2147/IJN.S69137
Enhancement of osseointegration of polyethylene terephthalate artificial ligament by coating of silk fibroin and depositing of hydroxyapatite
Abstract
Background: Application of artificial ligament in anterior cruciate ligament reconstruction is one of the research focuses of sports medicine but the biological tendon-bone healing still remains a problem. The preliminary study of hydroxyapatite (HAP) coating on the polyethylene terephthalate (PET) surface could effectively induce the osteoblast differentiation, but the tendon-bone healing was still not stable. As a green synthesis process, the biomimetic mineralization can simulate the natural bone growth in vitro and in vivo.
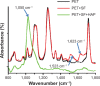
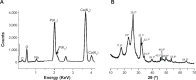
Methods: HAP crystals were grown under the guide of silk fibroin (SF) PET surface by biomimetic route. Several techniques including scanning electron microscopy, attenuated total reflectance Fourier transform infrared spectroscopy, X-ray diffraction, and energy-dispersive X-ray spectroscopy were utilized for proving the introduction of both SF and HAP. The viability and osseointegration of bone marrow stromal cells on the surface of three kinds of ligament, including PET group (non-coating group), PET+SF group (SF-coating group), and PET+SF+HAP group (combined HAP- and SF-coating group), were analyzed by CCK-8 assays and alkaline phosphatase (ALP) detection. Seventy-two mature male New Zealand rabbits were randomly divided into three groups. Among them, 36 rabbits were sacrificed for mechanical testing, and histological examination for the others.
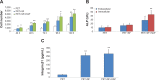
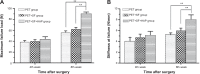
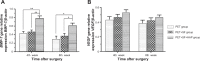
Results: The SF and SF+HAP were successfully coated on the surface of PET fiber. The CCK-8 assay showed that the cell proliferation on PET+SF+HAP group was better than the other two groups from 24 to 120 hours. After 14 days of culture, the cells in the PET+SF+HAP group delivered higher levels of ALP than the other two groups. After 3 days of culture, the expression level of integrin β1 in the PET+SF+HAP group and PET+SF group were higher than in the PET group. The mean load to failure and the stiffness value of the PET+SF+HAP group were both higher than the other two groups. Hematoxylin and eosin staining showed that new bone tissue formation was only found in the PET+SF+HAP group 8 weeks postoperatively. Masson staining showed that in the PET+SF+HAP group 8 weeks postoperatively, the PET fibers were almost completely encircled by collagen. Histomorphometric analysis showed that the width of the graft-bone interface in the PET+SF+HAP group was narrower than that in the other two groups 4 and 8 weeks postoperatively. The mRNA level of BMP-7 in the PET+SF+HAP groups was significantly higher than those in the other two groups 4 and 8 weeks postoperatively.
Conclusion: The study showed that the combined SF and HAP coating by biomimetic route on the surface of PET artificial ligament could induce graft osseointegration in the bone tunnel, providing theoretical and experimental foundation for manufacturing novel artificial ligaments meeting the clinical needs.
Keywords: biomineralization; ligament reconstruction; tendon–bone healing.
Figures




References
-
- Guarino V, Causa F, Ambrosio L. Bioactive scaffolds for bone and ligament tissue. Expert Rev Med Devices. 2007;4(3):405–418. - PubMed
-
- Harner CD, Giffin JR, Dunteman RC, Annunziata CC, Friedman MJ. Evaluation and treatment of recurrent instability after anterior cruciate ligament reconstruction. Instr Course Lect. 2001;50:463–474. - PubMed
-
- Heath CA, Rutkowski GE. The development of bioartificial nerve grafts for peripheral-nerve regeneration. Trends Biotechnol. 1998;16(4):163–168. - PubMed
-
- Brunelli GA, Battiston B, Vigasio A, Brunelli G, Marocolo D. Bridging nerve defects with combined skeletal muscle and vein conduits. Microsurgery. 1993;14(4):247–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

