An evidence-based review of ixazomib citrate and its potential in the treatment of newly diagnosed multiple myeloma
- PMID: 25302026
- PMCID: PMC4189713
- DOI: 10.2147/OTT.S49187
An evidence-based review of ixazomib citrate and its potential in the treatment of newly diagnosed multiple myeloma
Abstract
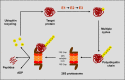
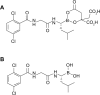
Proteasome inhibition represents one of the more important therapeutic targets in the treatment of multiple myeloma (MM), since by suppressing nuclear factor-κB activity, which promotes myelomagenesis, it makes plasma cells susceptible to proapoptotic signals. Bortezomib, the first proteasome inhibitor approved for MM therapy, has been shown to increase response rate and improve outcome in patients with relapsed/refractory disease and in the frontline setting, particularly when combined with immunomodulatory drugs and alkylating agents. Among second-generation proteasome inhibitors, ixazomib (MLN9708) is the first oral compound to be evaluated for the treatment of MM. Ixazomib has shown improved pharmacokinetic and pharmacodynamic parameters compared with bortezomib, in addition to similar efficacy in the control of myeloma growth and prevention of bone loss. Ixazomib was found to overcome bortezomib resistance and to trigger synergistic antimyeloma activity with dexamethasone, lenalidomide, and histone deacetylase inhibitors. Phase I/II studies using ixazomib weekly or twice weekly in relapsed/refractory MM patients suggested antitumor activity of the single agent, but more promising results have been obtained with the combination of ixazomib, lenalidomide, and dexamethasone in newly diagnosed MM. Ixazomib has also been used in systemic amyloidosis as a single agent, showing important activity in this difficult-to-treat plasma-cell dyscrasia. More frequent side effects observed during administration of ixazomib were thrombocytopenia, nausea, vomiting, diarrhea, fatigue, and rash, whereas severe peripheral neuropathy was rare. Here, we review the chemical characteristics of ixazomib, as well as its mechanism of action and results from preclinical and clinical trials.
Keywords: MLN9708; ixazomib; multiple myeloma; proteasome inhibitors.
Figures
References
-
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79(1):13–21. - PubMed
-
- Groll M, Ditzel L, Löwe J, et al. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature. 1997;386(6624):463–471. - PubMed
-
- Orlowski M, Wilk S. Catalytic activities of the 20S proteasome, a multicatalytic proteinase complex. Arch Biochem Biophys. 2000;383(1):1–16. - PubMed
-
- Rechsteiner MC. Ubiquitin-mediated proteolysis: an ideal pathway for systems biology analysis. Adv Exp Med Biol. 2004;547:49–59. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources