Pneumothorax as adverse event in patients with lung metastases of soft tissue sarcoma treated with pazopanib: a single reference centre case series
- PMID: 25302110
- PMCID: PMC4191680
- DOI: 10.1186/2045-3329-4-14
Pneumothorax as adverse event in patients with lung metastases of soft tissue sarcoma treated with pazopanib: a single reference centre case series
Abstract
Background: Recently, the phase III PALETTE study introduced pazopanib (Votrient®) as treatment for adult patients with locally advanced or metastatic non-liposarcoma soft tissue sarcoma after prior treatment with doxorubicin and/or ifosfamide. Pneumothorax was reported as adverse event in 8 of 246 treated patients (3.3%) in that study. This case series presents the incidence and clinic of this complication in the Leiden University Medical Centre.
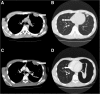
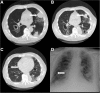
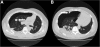
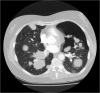
Cases: Forty-three patients were treated with pazopanib of which six patients (14.0%) developed a pneumothorax. These six patients were treated for malignant peripheral nerve sheath tumour, angiosarcoma, synovial sarcoma, fibromyxomatoid sarcoma, pleomorphic sarcoma and endometrial stromal sarcoma. All six patients had subpleural pulmonary or pleural metastases at the start of pazopanib and the pneumothorax developed during or shortly after treatment with pazopanib and was difficult to treat.
Discussion: The incidence reported by us is higher than the incidence in the PALETTE study. Trials with pazopanib in renal cell carcinoma, urothelial carcinoma and cervix carcinoma did not report pneumothorax as an adverse event, suggesting pneumothorax as a specific adverse event in soft tissue sarcoma patients treated with pazopanib. This may be related to the fact that there is often pleural metastatic involvement and cystic degeneration due to pazopanib treatment may add to the risk.
Conclusion: The risk of an, often difficult to treat, pneumothorax during pazopanib therapy should be discussed with the patient before initiation of treatment for a pulmonary metastasized sarcoma and physicians should be alert to the occurrence of such an event.
Keywords: Adverse event; Pazopanib; Pleural metastases; Pneumothorax; Pulmonary metastases; Soft tissue sarcoma.
Figures


References
-
- ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii92–vii99. - PubMed
-
- Le Cesne A, Blay JY, Judson I, Van Oosterom A, Verweij J, Radford J, Lorigan P, Rodenhuis S, Ray-Coquard I, Bonvalot S, Collin F, Jimeno J, Di Paola E, Van Glabbeke M, Nielsen OS. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol. 2005;23(3):576–584. - PubMed
-
- Demetri GD, Chawla SP, von Mehren M, Ritch P, Baker LH, Blay JY, Hande KR, Keohan ML, Samuels BL, Schuetze S, Lebedinsky C, Elsayed YA, Izquierdo MA, Gomez J, Park YC, Le Cesne A. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol. 2009;27(25):4188–4196. doi: 10.1200/JCO.2008.21.0088. - DOI - PubMed
-
- van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, Schoffski P, Aglietta M, Staddon AP, Beppu Y, Le Cesne A, Gelderblom H, Judson IR, Araki N, Ouali M, Marreaud S, Hodge R, Dewji MR, Coens C, Demetri GD, Fletcher CD, Dei Tos AP, Hohenberger P. EORTC Soft Tissue and Bone Sarcoma Group; PALETTE study group. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–1886. doi: 10.1016/S0140-6736(12)60651-5. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

