Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis
- PMID: 25302625
- PMCID: PMC5638117
- DOI: 10.1002/path.4462
Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis
Abstract
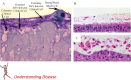
Infants and young children with acute onset of wheezing and reduced respiratory airflows are often diagnosed with obstruction and inflammation of the small bronchiolar airways, ie bronchiolitis. The most common aetological agents causing bronchiolitis in young children are the respiratory viruses, and of the commonly encountered respiratory viruses, respiratory syncytial virus (RSV) has a propensity for causing bronchiolitis. Indeed, RSV bronchiolitis remains the major reason why previously healthy infants are admitted to hospital. Why RSV infection is such a predominant cause of bronchiolitis is the subject of this review. By reviewing the available histopathology of RSV bronchiolitis, both in humans and relevant animal models, we identify hallmark features of RSV infection of the distal airways and focus attention on the consequences of columnar cell cytopathology occurring in the bronchioles, which directly impacts the development of bronchiolar obstruction, inflammation and disease.
Keywords: acute lung disease; airway obstruction; bronchiolitis; respiratory syncytial virus.
Copyright © 2014 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Figures


References
-
- Wright AL, Taussig LM, Ray CG, et al. The Tucson Children's Respiratory Study. II. Lower respiratory tract illness in the first year of life. Am J Epidemiol 1989; 129: 1232–1246. - PubMed
-
- Glezen WP, Taber LH, Frank AL, et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140: 543–546. - PubMed
-
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med 2001; 344: 1917–1928. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

