Ethylphenidate as a selective dopaminergic agonist and methylphenidate-ethanol transesterification biomarker
- PMID: 25303048
- PMCID: PMC4237660
- DOI: 10.1002/jps.24202
Ethylphenidate as a selective dopaminergic agonist and methylphenidate-ethanol transesterification biomarker
Abstract
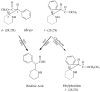
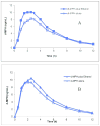
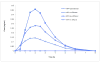
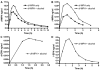
We review the pharmaceutical science of ethylphenidate (EPH) in the contexts of drug discovery, drug interactions, biomarker for dl-methylphenidate (MPH)-ethanol exposure, potentiation of dl-MPH abuse liability, contemporary "designer drug," pertinence to the newer transdermal and chiral switch MPH formulations, as well as problematic internal standard. d-EPH selectively targets the dopamine transporter, whereas d-MPH exhibits equipotent actions at dopamine and norepinephrine transporters. This selectivity carries implications for the advancement of tailored attention-deficit/hyperactivity disorder (ADHD) pharmacotherapy in the era of genome-based diagnostics. Abuse of dl-MPH often involves ethanol coabuse. Carboxylesterase 1 enantioselectively transesterifies l-MPH with ethanol to yield l-EPH accompanied by significantly increased early exposure to d-MPH and rapid potentiation of euphoria. The pharmacokinetic component of this drug interaction can largely be avoided using dexmethylphenidate (dexMPH). This notwithstanding, maximal potentiated euphoria occurs following dexMPH-ethanol. C57BL/6 mice model dl-MPH-ethanol interactions: an otherwise depressive dose of ethanol synergistically increases dl-MPH stimulation; a substimulatory dose of dl-MPH potentiates a low, stimulatory dose of ethanol; ethanol elevates blood, brain, and urinary d-MPH concentrations while forming l-EPH. Integration of EPH preclinical neuropharmacology with clinical studies of MPH-ethanol interactions provides a translational approach toward advancement of ADHD personalized medicine and management of comorbid alcohol use disorder.
Keywords: absorption; bioavailability; dexmethylphenidate; drug interaction; ethanol; ethylphenidate; metabolism; methylphenidate; pharmacokinetics/pharmacodynamics; transesterification.
© 2014 Wiley Periodicals, Inc. and the American Pharmacists Association.
Conflict of interest statement
K.S. Patrick serves as a consultant for Noven, Alza, UCB and Shire and Ortho-Janssen. He has served as a consultant to Johnson & Johnson and Celgene within the last 5 years and has had a provisional patent for isopropylphenidate (ritalinic acid isopropyl ester) as a novel psychotropic agent through the MUSC Foundation for Research Development, with a Notice of abandonment Jan 2014. No other activities of the authors could be construed as conflicts.
Figures
References
-
- Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002-2010. Pediatrics. 2012;130(1):23–31. - PubMed
-
- Upadhyaya HP. Managing attention-deficit/hyperactivity disorder in the presence of substance use disorder. J Clin Psychiatry. 2007;68(Suppl 11):23–30. - PubMed
-
- NSDUH National Survey on Drug Use and Health. 2012 Pub id SMA13-4796.
-
- Darredeau C, Barrett SP, Jardin B, Pihl RO. Patterns and predictors of medication compliance, diversion, and misuse in adult prescribed methylphenidate users. Hum Psychopharmacol. 2007;22:529–536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

