γCaMKII shuttles Ca²⁺/CaM to the nucleus to trigger CREB phosphorylation and gene expression
- PMID: 25303525
- PMCID: PMC4201038
- DOI: 10.1016/j.cell.2014.09.019
γCaMKII shuttles Ca²⁺/CaM to the nucleus to trigger CREB phosphorylation and gene expression
Abstract
Activity-dependent CREB phosphorylation and gene expression are critical for long-term neuronal plasticity. Local signaling at CaV1 channels triggers these events, but how information is relayed onward to the nucleus remains unclear. Here, we report a mechanism that mediates long-distance communication within cells: a shuttle that transports Ca(2+)/calmodulin from the surface membrane to the nucleus. We show that the shuttle protein is γCaMKII, its phosphorylation at Thr287 by βCaMKII protects the Ca(2+)/CaM signal, and CaN triggers its nuclear translocation. Both βCaMKII and CaN act in close proximity to CaV1 channels, supporting their dominance, whereas γCaMKII operates as a carrier, not as a kinase. Upon arrival within the nucleus, Ca(2+)/CaM activates CaMKK and its substrate CaMKIV, the CREB kinase. This mechanism resolves long-standing puzzles about CaM/CaMK-dependent signaling to the nucleus. The significance of the mechanism is emphasized by dysregulation of CaV1, γCaMKII, βCaMKII, and CaN in multiple neuropsychiatric disorders.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
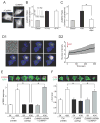
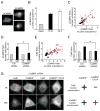

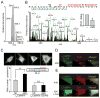

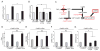

Comment in
-
Mission CaMKIIγ: shuttle calmodulin from membrane to nucleus.Cell. 2014 Oct 9;159(2):235-7. doi: 10.1016/j.cell.2014.09.023. Cell. 2014. PMID: 25303520 Free PMC article.
References
-
- Bachs O, Agell N, Carafoli E. Calmodulin and calmodulin-binding proteins in the nucleus. Cell calcium. 1994;16:289–296. - PubMed
-
- Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. - PubMed
-
- Baudier J, Deloulme JC, Van Dorsselaer A, Black D, Matthes HW. Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17) J Biol Chem. 1991;266:229–237. - PubMed
-
- Bayer KU, Lohler J, Schulman H, Harbers K. Developmental expression of the CaM kinase II isoforms: ubiquitous gamma- and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain Res Mol Brain Res. 1999;70:147–154. - PubMed
-
- Berridge MJ. Calcium signalling remodelling and disease. Biochem Soc Trans. 2012;40:297–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

