Generation of functional human pancreatic β cells in vitro
- PMID: 25303535
- PMCID: PMC4617632
- DOI: 10.1016/j.cell.2014.09.040
Generation of functional human pancreatic β cells in vitro
Abstract
The generation of insulin-producing pancreatic β cells from stem cells in vitro would provide an unprecedented cell source for drug discovery and cell transplantation therapy in diabetes. However, insulin-producing cells previously generated from human pluripotent stem cells (hPSC) lack many functional characteristics of bona fide β cells. Here, we report a scalable differentiation protocol that can generate hundreds of millions of glucose-responsive β cells from hPSC in vitro. These stem-cell-derived β cells (SC-β) express markers found in mature β cells, flux Ca(2+) in response to glucose, package insulin into secretory granules, and secrete quantities of insulin comparable to adult β cells in response to multiple sequential glucose challenges in vitro. Furthermore, these cells secrete human insulin into the serum of mice shortly after transplantation in a glucose-regulated manner, and transplantation of these cells ameliorates hyperglycemia in diabetic mice.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
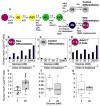

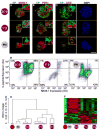

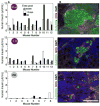
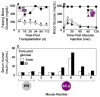
Comment in
-
Diabetes: β cells at last.Nat Rev Endocrinol. 2015 Jan;11(1):5-6. doi: 10.1038/nrendo.2014.200. Epub 2014 Nov 11. Nat Rev Endocrinol. 2015. PMID: 25385037 No abstract available.
-
Stem cells: Insulin-producing β cells in a dish.Nat Rev Mol Cell Biol. 2014 Dec;15(12):768. doi: 10.1038/nrm3907. Epub 2014 Nov 12. Nat Rev Mol Cell Biol. 2014. PMID: 25387398 No abstract available.
-
'Diabetes: a cure at last' or just hype?Diabet Med. 2014 Dec;31(12):1479. doi: 10.1111/dme.12611. Diabet Med. 2014. PMID: 25399773 No abstract available.
References
-
- Aguayo-Mazzucato C, Zavacki AM, Marinelarena A, Hollister-Lock J, Khattabi IE, Marsili A, Weir GC, Sharma A, Larsen PR, Bonner-Weir S. Thyroid Hormone Promotes Postnatal Rat Pancreatic β-Cell Development and Glucose-Responsive Insulin Secretion Through MAFA. Diabetes. 2013;62:1569–580. - PMC - PubMed
-
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Nature. 1999;400:877–881. - PubMed
-
- Bellin MD, Barton FB, Heitman A, Harmon J, Balamurugan AN, Kandaswamy R, Sutherland DE, Alejandro R, Hering BJ. Potent Induction Immunotherapy Promotes Long-Term Insulin Independence After Islet Transplantation in Type 1 Diabetes. American Journal of Transplantation. 2012;12:1576–583. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

