HIV, hepatitis B virus, and hepatitis C virus co-infection in patients in the China National Free Antiretroviral Treatment Program, 2010-12: a retrospective observational cohort study
- PMID: 25303841
- PMCID: PMC6051428
- DOI: 10.1016/S1473-3099(14)70946-6
HIV, hepatitis B virus, and hepatitis C virus co-infection in patients in the China National Free Antiretroviral Treatment Program, 2010-12: a retrospective observational cohort study
Abstract
Background: Hepatitis-related liver diseases are a leading cause of mortality and morbidity among people with HIV/AIDS taking combination antiretroviral therapy. We assessed the effect of hepatitis B virus (HBV) and hepatitis C virus (HCV) co-infection on HIV outcomes in patients in China.
Methods: We did a nationwide retrospective observational cohort study with data from the China National Free Antiretroviral Treatment Program from 2010-11. Patients older than 18 years starting standard antiretroviral therapy for HIV who had tested positive for HBV and HCV were followed up to Dec 31, 2012. We used Kaplan-Meier analysis and Cox proportional hazard models to evaluate survival, and logistic regression models to estimate virological failure, immunological response, and retention in care.
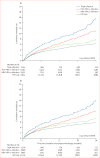
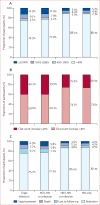
Findings: 33 861 patients with HIV met eligibility criteria. 2958 (8·7%) participants had HBV co-infection, 6149 (18·2%) had HCV co-infection, and 1114 (3·3%) had triple infection. All-cause mortality was higher in participants with triple infection (adjusted hazard ratio 1·90, 95% CI 1·53-2·37) and HCV co-infection (1·46, 1·25-1·70) than in those with HIV only, but not in those with HBV co-infection (1·06, 0·89-1·26). People with triple infection were also more likely to have virological failure (adjusted odds ratio [OR] 1·26, 95% CI 1·02-1·56) than were those with HIV only, whereas the difference was not significant for those with HBV co-infection (0·93, 0·80-1·10) or HCV co-infection (1·10, 0·97-1·26). No co-infection was significantly associated with a difference in CD4 cell count after 1 year of treatment. Loss to follow-up was more common among participants with triple infection (OR 1·37, 95% CI 1·16-1·62) and HCV co-infection (1·30, 1·17-1·45), but not HBV co-infection (0·93, 0·82-1·05), than among those with HIV only.
Interpretation: Screening for viral hepatitis is important in individuals diagnosed as HIV positive. Effective management for viral hepatitis should be integrated into HIV treatment programmes. Long-term data are needed about the effect of hepatitis co-infection on HIV disease progression.
Funding: The National Center for AIDS/STD Control and Prevention, China Center for Disease Control and Prevention.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
We declare no competing interests.
Figures

Comment in
-
Hepatitis virus and HIV interactions.Lancet Infect Dis. 2014 Nov;14(11):1025-1027. doi: 10.1016/S1473-3099(14)70853-9. Epub 2014 Oct 7. Lancet Infect Dis. 2014. PMID: 25303842 No abstract available.
References
-
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67. - PubMed
-
- Easterbrook P, Sands A, Harmanci H. Challenges and priorities in the management of HIV/HBV and HIV/HCV coinfection in resource-limited settings. Semin Liver Dis. 2012;32:147–57. - PubMed
-
- UNAIDS. [accessed Aug 26, 2014];Global report: UNAIDS report on the global AIDS epidemic. 2012 http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiolo....
-
- Barth RE, Huijgen Q, Taljaard J, Hoepelman AI. Hepatitis B/C and HIV in sub-Saharan Africa: an association between highly prevalent infectious diseases. A systematic review and meta-analysis. Int J Infect Dis. 2010;14:e1024–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

