Crosstalk between Mycobacterium tuberculosis and the host cell
- PMID: 25303934
- PMCID: PMC4250340
- DOI: 10.1016/j.smim.2014.09.002
Crosstalk between Mycobacterium tuberculosis and the host cell
Abstract
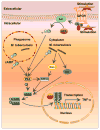
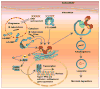
The successful establishment and maintenance of a bacterial infection depend on the pathogen's ability to subvert the host cell's defense response and successfully survive, proliferate, or persist within the infected cell. To circumvent host defense systems, bacterial pathogens produce a variety of virulence factors that potentiate bacterial adherence and invasion and usurp host cell signaling cascades that regulate intracellular microbial survival and trafficking. Mycobacterium tuberculosis, probably one of the most successful pathogens on earth, has coexisted with humanity for centuries, and this intimate and persistent connection between these two organisms suggests that the pathogen has evolved extensive mechanisms to evade the human immune system at multiple levels. While some of these mechanisms are mediated by factors released by M. tuberculosis, others rely on host components that are hijacked to prevent the generation of an effective immune response thus benefiting the survival of M. tuberculosis within the host cell. Here, we describe several of these mechanisms, with an emphasis on the cyclic nucleotide signaling and subversion of host responses that occur at the intracellular level when tubercle bacilli encounter macrophages, a cell that becomes a safe-house for M. tuberculosis although it is specialized to kill most microbes.
Keywords: Cyclic AMP; Cyclic di-AMP; Immunity; Interferon; Macrophage; Mycobacterium tuberculosis.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Host-pathogen interactions during Mycobacterium tuberculosis infections.Curr Top Microbiol Immunol. 2013;374:211-41. doi: 10.1007/82_2013_332. Curr Top Microbiol Immunol. 2013. PMID: 23881288 Review.
-
Macrophage immunoregulatory pathways in tuberculosis.Semin Immunol. 2014 Dec;26(6):471-85. doi: 10.1016/j.smim.2014.09.010. Epub 2014 Oct 30. Semin Immunol. 2014. PMID: 25453226 Free PMC article. Review.
-
Understanding delayed T-cell priming, lung recruitment, and airway luminal T-cell responses in host defense against pulmonary tuberculosis.Clin Dev Immunol. 2012;2012:628293. doi: 10.1155/2012/628293. Epub 2012 Apr 1. Clin Dev Immunol. 2012. PMID: 22545059 Free PMC article. Review.
-
Cell death and autophagy in tuberculosis.Semin Immunol. 2014 Dec;26(6):497-511. doi: 10.1016/j.smim.2014.10.001. Epub 2014 Oct 17. Semin Immunol. 2014. PMID: 25453227 Free PMC article. Review.
-
Mycobacterium tuberculosis with different virulence reside within intact phagosomes and inhibit phagolysosomal biogenesis in alveolar macrophages of patients with pulmonary tuberculosis.Tuberculosis (Edinb). 2019 Jan;114:77-90. doi: 10.1016/j.tube.2018.12.002. Epub 2018 Dec 6. Tuberculosis (Edinb). 2019. PMID: 30711161
Cited by
-
Opsonic monoclonal antibodies enhance phagocytic killing activity and clearance of Mycobacterium tuberculosis from blood in a quantitative qPCR mouse model.Heliyon. 2019 Sep 6;5(9):e02260. doi: 10.1016/j.heliyon.2019.e02260. eCollection 2019 Sep. Heliyon. 2019. PMID: 31517107 Free PMC article.
-
Structural and Biochemical Insight into the Mechanism of Rv2837c from Mycobacterium tuberculosis as a c-di-NMP Phosphodiesterase.J Biol Chem. 2016 Feb 12;291(7):3668-81. doi: 10.1074/jbc.M115.699801. Epub 2015 Dec 14. J Biol Chem. 2016. PMID: 26668313 Free PMC article.
-
EBP50 induces apoptosis in macrophages by upregulating nitric oxide production to eliminate intracellular Mycobacterium tuberculosis.Sci Rep. 2016 Jan 5;6:18961. doi: 10.1038/srep18961. Sci Rep. 2016. PMID: 26729618 Free PMC article.
-
Immunometabolism in Tuberculosis.Front Immunol. 2016 Apr 21;7:150. doi: 10.3389/fimmu.2016.00150. eCollection 2016. Front Immunol. 2016. PMID: 27148269 Free PMC article. Review.
-
Activation of transcription factor CREB in human macrophages by Mycobacterium tuberculosis promotes bacterial survival, reduces NF-kB nuclear transit and limits phagolysosome fusion by reduced necroptotic signaling.PLoS Pathog. 2023 Mar 31;19(3):e1011297. doi: 10.1371/journal.ppat.1011297. eCollection 2023 Mar. PLoS Pathog. 2023. PMID: 37000865 Free PMC article.
References
-
- Jayachandran R, BoseDasgupta S, Pieters J. Surviving the macrophage: tools and tricks employed by Mycobacterium tuberculosis. Curr Top Microbiol Immunol. 2013;374:189–209. - PubMed
-
- Stanley SA, Cox JS. Host-pathogen interactions during Mycobacterium tuberculosis infections. Curr Top Microbiol Immunol. 2013;374:211–41. - PubMed
-
- Stokes RW, Thorson LM, Speert DP. Nonopsonic and opsonic association of Mycobacterium tuberculosis with resident alveolar macrophages is inefficient. J Immunol. 1998;160(11):5514–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

