Activation and propagation of tumor-infiltrating lymphocytes on clinical-grade designer artificial antigen-presenting cells for adoptive immunotherapy of melanoma
- PMID: 25304728
- PMCID: PMC4199393
- DOI: 10.1097/CJI.0000000000000056
Activation and propagation of tumor-infiltrating lymphocytes on clinical-grade designer artificial antigen-presenting cells for adoptive immunotherapy of melanoma
Abstract
Purpose: Adoptive cell therapy with autologous tumor-infiltrating lymphocytes (TIL) is a therapy for metastatic melanoma with response rates of up to 50%. However, the generation of the TIL transfer product is challenging, requiring pooled allogeneic normal donor peripheral blood mononuclear cells (PBMC) used in vitro as "feeders" to support a rapid-expansion protocol. Here, we optimized a platform to propagate TIL to a clinical scale using K562 cells genetically modified to express costimulatory molecules such as CD86, CD137-ligand, and membrane-bound IL-15 to function as artificial antigen-presenting cells (aAPC) as an alternative to using PBMC feeders.
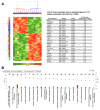
Experimental design: We used aAPC or γ-irradiated PBMC feeders to propagate TIL and measured rates of expansion. The activation and differentiation state was evaluated by flow cytometry and differential gene expression analyses. Clonal diversity was assessed on the basis of the pattern of T-cell receptor usage. T-cell effector function was measured by evaluation of cytotoxic granule content and killing of target cells.
Results: The aAPC propagated TIL at numbers equivalent to that found with PBMC feeders, whereas increasing the frequency of CD8 T-cell expansion with a comparable effector-memory phenotype. mRNA profiling revealed an upregulation of genes in the Wnt and stem-cell pathways with the aAPC. The aAPC platform did not skew clonal diversity, and CD8 T cells showed comparable antitumor function as those expanded with PBMC feeders.
Conclusions: TIL can be rapidly expanded with aAPC to clinical scale generating T cells with similar phenotypic and effector profiles as with PBMC feeders. These data support the clinical application of aAPC to manufacture TIL for the treatment of melanoma.
Figures





References
-
- Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655. - PubMed
-
- Itzhaki O, Hovav E, Ziporen Y, et al. Establishment and large-scale expansion of minimally cultured “young” tumor infiltrating lymphocytes for adoptive transfer therapy. J Immunother. 2011;34:212–220. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

