The impact of chronic migraine: The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study methods and baseline results
- PMID: 25304766
- PMCID: PMC4430584
- DOI: 10.1177/0333102414552532
The impact of chronic migraine: The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study methods and baseline results
Abstract
Background: Longitudinal migraine studies have rarely assessed headache frequency and disability variation over a year.
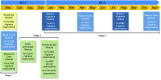
Methods: The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study is a cross-sectional and longitudinal Internet study designed to characterize the course of episodic migraine (EM) and chronic migraine (CM). Participants were recruited from a Web-panel using quota sampling in an attempt to obtain a sample demographically similar to the US population. Participants who passed the screener were assessed every three months with the Core (baseline, six, and 12 months) and Snapshot (months three and nine) modules, which assessed headache frequency, headache-related disability, treatments, and treatment satisfaction. The Core also assessed resource use, health-related quality of life, and other features. One-time cross-sectional modules measured family burden, barriers to medical care, and comorbidities/endophenotypes.
Results: Of 489,537 invitees, we obtained 58,418 (11.9%) usable returns including 16,789 individuals who met ICHD-3 beta migraine criteria (EM (<15 headache days/mo): n = 15,313 (91.2%); CM (≥ 15 headache days/mo): n = 1476 (8.8%)). At baseline, all qualified respondents (n = 16,789) completed the Screener, Core, and Barriers to Care modules. Subsequent modules showed some attrition (Comorbidities/Endophenotypes, n = 12,810; Family Burden (Proband), n = 13,064; Family Burden (Partner), n = 4022; Family Burden (Child), n = 2140; Snapshot (three months), n = 9741; Core (six months), n = 7517; Snapshot (nine months), n = 6362; Core (12 months), n = 5915). A total of 3513 respondents (21.0%) completed all modules, and 3626 (EM: n = 3303 (21.6%); CM: n = 323 (21.9%)) completed all longitudinal assessments.
Conclusions: The CaMEO Study provides cross-sectional and longitudinal data that will contribute to our understanding of the course of migraine over one year and quantify variations in headache frequency, headache-related disability, comorbidities, treatments, and familial impact.
Trial registration: ClinicalTrials.gov NCT01648530.
Keywords: Migraine disorders; chronic migraine; epidemiology; episodic migraine; headache.
© International Headache Society 2014 Reprints and permissions: sagepub.co.uk/journalsPermissions.nav.
Figures

References
-
- Victor TW, Hu X, Campbell JC, et al. Migraine prevalence by age and sex in the United States: A life-span study. Cephalalgia 2010; 30: 1065–1072. - PubMed
-
- Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007; 68: 343–349. - PubMed
-
- Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: Results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2013; 53: 1278–1299. - PubMed
-
- Manack A, Turkel C, Silberstein S. The evolution of chronic migraine: Classification and nomenclature. Headache 2009; 49: 1206–1213. - PubMed
-
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

