Aromatic anchor at an invariant hormone-receptor interface: function of insulin residue B24 with application to protein design
- PMID: 25305014
- PMCID: PMC4263875
- DOI: 10.1074/jbc.M114.608562
Aromatic anchor at an invariant hormone-receptor interface: function of insulin residue B24 with application to protein design
Abstract
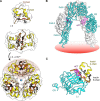
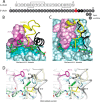
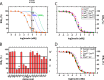
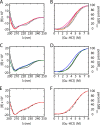
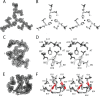
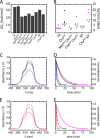
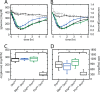
Crystallographic studies of insulin bound to fragments of the insulin receptor have recently defined the topography of the primary hormone-receptor interface. Here, we have investigated the role of Phe(B24), an invariant aromatic anchor at this interface and site of a human mutation causing diabetes mellitus. An extensive set of B24 substitutions has been constructed and tested for effects on receptor binding. Although aromaticity has long been considered a key requirement at this position, Met(B24) was found to confer essentially native affinity and bioactivity. Molecular modeling suggests that this linear side chain can serve as an alternative hydrophobic anchor at the hormone-receptor interface. These findings motivated further substitution of Phe(B24) by cyclohexanylalanine (Cha), which contains a nonplanar aliphatic ring. Contrary to expectations, [Cha(B24)]insulin likewise exhibited high activity. Furthermore, its resistance to fibrillation and the rapid rate of hexamer disassembly, properties of potential therapeutic advantage, were enhanced. The crystal structure of the Cha(B24) analog, determined as an R6 zinc-stabilized hexamer at a resolution of 1.5 Å, closely resembles that of wild-type insulin. The nonplanar aliphatic ring exhibits two chair conformations with partial occupancies, each recapitulating the role of Phe(B24) at the dimer interface. Together, these studies have defined structural requirements of an anchor residue within the B24-binding pocket of the insulin receptor; similar molecular principles are likely to pertain to insulin-related growth factors. Our results highlight in particular the utility of nonaromatic side chains as probes of the B24 pocket and suggest that the nonstandard Cha side chain may have therapeutic utility.
Keywords: Diabetes; Hormone; Mutagenesis; Protein Design; Protein Structure; Receptor Tyrosine Kinase; nonstandard mutagenesis.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

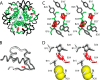


References
-
- De Meyts P., Whittaker J. (2002) Structural biology of insulin and IGF1 receptors: implications for drug design. Nat. Rev. Drug Discov. 1, 769–783 - PubMed
-
- Baker E. N., Blundell T. L., Cutfield J. F., Cutfield S. M., Dodson E. J., Dodson G. G., Hodgkin D. M., Hubbard R. E., Isaacs N. W., Reynolds C. D. (1988) The structure of 2Zn pig insulin crystals at 1.5 Å resolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 319, 369–456 - PubMed
-
- McKern N. M., Lawrence M. C., Streltsov V. A., Lou M. Z., Adams T. E., Lovrecz G. O., Elleman T. C., Richards K. M., Bentley J. D., Pilling P. A., Hoyne P. A., Cartledge K. A., Pham T. M., Lewis J. L., Sankovich S. E., Stoichevska V., Da Silva E., Robinson C. P., Frenkel M. J., Sparrow L. G., Fernley R. T., Epa V. C., Ward C. W. (2006) Structure of the insulin receptor ectodomain reveals a folded-over conformation. Nature 443, 218–221 - PubMed
-
- Smith B. J., Huang K., Kong G., Chan S. J., Nakagawa S., Menting J. G., Hu S.-Q., Whittaker J., Steiner D. F., Katsoyannis P. G., Ward C. W., Weiss M. A., Lawrence M. C. (2010) Structural resolution of a tandem hormone-binding element in the insulin receptor and its implications for design of peptide agonists. Proc. Natl. Acad. Sci. U.S.A. 107, 6771–6776 - PMC - PubMed
-
- Menting J. G., Whittaker J., Margetts M. B., Whittaker L. J., Kong G. K., Smith B. J., Watson C. J., Záková L., Kletvíková E., Jirácek J., Chan S. J., Steiner D. F., Dodson G. G., Brzozowski A. M., Weiss M. A., Ward C. W., Lawrence M. C. (2013) How insulin engages its primary binding site on the insulin receptor. Nature 493, 241–245 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

