Assessing quality of life on the day of chemotherapy administration underestimates patients' true symptom burden
- PMID: 25305067
- PMCID: PMC4198707
- DOI: 10.1186/1471-2407-14-758
Assessing quality of life on the day of chemotherapy administration underestimates patients' true symptom burden
Abstract
Background: In chemotherapy trials quality of life (QOL) is assessed mostly at the days of chemotherapy administration (i.e. event-driven) during treatment and follows fixed time intervals in the aftercare phase (i.e. time-driven). Specific QOL impairments and treatment side-effects are known to be time dependent following different trajectories. Therefore, acute problems are likely to be missed if assessments are done infrequently or at inappropriate time points. Since the planning of supportive care interventions during chemotherapy depends on knowledge about symptom trajectories, such information may be of substantial importance to a clinician.
Methods: Cancer patients receiving chemotherapy at Kufstein County Hospital were assessed using an electronic version of the EORTC QLQ-C30 at the day of chemotherapy administration at the hospital. One and two weeks later assessments were repeated via the internet while patients were at home. Assessments at home and the hospital were conducted using the web-based software CHES. Data were analysed by means of linear mixed models.
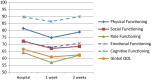
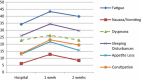
Results: A sample of 54 chemotherapy outpatients participated in electronic QOL assessments at the hospital and at home. For 9 out of the 15 QOL domains of the EORTC QLQ-C30 patients reported increased burden one week after chemotherapy administration compared to the day of chemotherapy administration. Most pronounced differences were found for Fatigue, Constipation, and Appetite Loss.
Conclusions: Our results indicate that patients experience most severe QOL impairments in the week following chemotherapy administration. This is a period that is usually not covered by QOL assessments in chemotherapy trials which may result in underestimation of true treatment burden. Our findings suggest to conduct QOL assessments not only event- or time-driven, but to rely on specific hypotheses on symptom and functioning trajectories.
Figures
References
-
- Fairclough D. Design and Analysis of Quality of Life Studies in Clinical Trials. Boca Raton: CRC Press; 2010.
-
- Ediebah DE, Coens C, Maringwa JT, Quinten C, Zikos E, Ringash J, King M, Gotay C, Flechtner HH, Schmucker von Koch J, Weis J, Smit EF, Kohne CH, Bottomley A. Effect of completion-time windows in the analysis of health-related quality of life outcomes in cancer patients. Ann Oncol. 2013;24(1):231–237. doi: 10.1093/annonc/mds220. - DOI - PMC - PubMed
-
- Pater J, Osoba D, Zee B, Lofters W, Gore M, Dempsey E, Palmer M, Chin C. Effects of altering the time of administration and the time frame of quality of life assessments in clinical trials: an example using the EORTC QLQ-C30 in a large anti-emetic trial. Qual Life Res. 1998;7(3):273–278. doi: 10.1023/A:1008886200225. - DOI - PubMed
-
- Hakamies-Blomqvist L, Luoma ML, Sjostrom J, Pluzanska A, Sjodin M, Mouridsen H, Ostenstad B, Mjaaland I, Ottosson S, Bergh J, Malmstrom PO, Blomqvist C. Timing of quality of life (QoL) assessments as a source of error in oncological trials. J Adv Nurs. 2001;35(5):709–716. doi: 10.1046/j.1365-2648.2001.01903.x. - DOI - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/758/prepub
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

