Rab11 modulates α-synuclein-mediated defects in synaptic transmission and behaviour
- PMID: 25305083
- PMCID: PMC4986550
- DOI: 10.1093/hmg/ddu521
Rab11 modulates α-synuclein-mediated defects in synaptic transmission and behaviour
Abstract
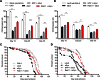
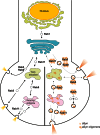
A central pathological hallmark of Parkinson's disease (PD) is the presence of proteinaceous depositions known as Lewy bodies, which consist largely of the protein α-synuclein (aSyn). Mutations, multiplications and polymorphisms in the gene encoding aSyn are associated with familial forms of PD and susceptibility to idiopathic PD. Alterations in aSyn impair neuronal vesicle formation/transport, and likely contribute to PD pathogenesis by neuronal dysfunction and degeneration. aSyn is functionally associated with several Rab family GTPases, which perform various roles in vesicle trafficking. Here, we explore the role of the endosomal recycling factor Rab11 in the pathogenesis of PD using Drosophila models of aSyn toxicity. We find that aSyn induces synaptic potentiation at the larval neuromuscular junction by increasing synaptic vesicle (SV) size, and that these alterations are reversed by Rab11 overexpression. Furthermore, Rab11 decreases aSyn aggregation and ameliorates several aSyn-dependent phenotypes in both larvae and adult fruit flies, including locomotor activity, degeneration of dopaminergic neurons and shortened lifespan. This work emphasizes the importance of Rab11 in the modulation of SV size and consequent enhancement of synaptic function. Our results suggest that targeting Rab11 activity could have a therapeutic value in PD.
© The Author 2014. Published by Oxford University Press.
Figures
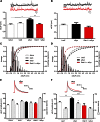

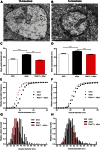
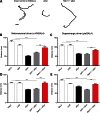
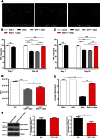
References
-
- Twelves D., Perkins K.S., Counsell C. Systematic review of incidence studies of Parkinson's disease. Mov. Disord. 2003;18:19–31. - PubMed
-
- de Lau L.M., Breteler M.M. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–535. - PubMed
-
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2001;2:492–501. - PubMed
-
- Crowther R.A., Daniel S.E., Goedert M. Characterisation of isolated alpha-synuclein filaments from substantia nigra of Parkinson's disease brain. Neurosci. Lett. 2000;292:128–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

