Force maintenance and myosin filament assembly regulated by Rho-kinase in airway smooth muscle
- PMID: 25305246
- PMCID: PMC4281697
- DOI: 10.1152/ajplung.00222.2014
Force maintenance and myosin filament assembly regulated by Rho-kinase in airway smooth muscle
Abstract
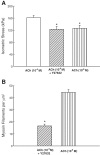
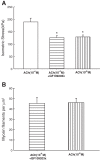
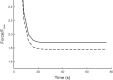
Smooth muscle contraction can be divided into two phases: the initial contraction determines the amount of developed force and the second phase determines how well the force is maintained. The initial phase is primarily due to activation of actomyosin interaction and is relatively well understood, whereas the second phase remains poorly understood. Force maintenance in the sustained phase can be disrupted by strains applied to the muscle; the strain causes actomyosin cross-bridges to detach and also the cytoskeletal structure to disassemble in a process known as fluidization, for which the underlying mechanism is largely unknown. In the present study we investigated the ability of airway smooth muscle to maintain force after the initial phase of contraction. Specifically, we examined the roles of Rho-kinase and protein kinase C (PKC) in force maintenance. We found that for the same degree of initial force inhibition, Rho-kinase substantially reduced the muscle's ability to sustain force under static conditions, whereas inhibition of PKC had a minimal effect on sustaining force. Under oscillatory strain, Rho-kinase inhibition caused further decline in force, but again, PKC inhibition had a minimal effect. We also found that Rho-kinase inhibition led to a decrease in the myosin filament mass in the muscle cells, suggesting that one of the functions of Rho-kinase is to stabilize myosin filaments. The results also suggest that dissolution of myosin filaments may be one of the mechanisms underlying the phenomenon of fluidization. These findings can shed light on the mechanism underlying deep inspiration induced bronchodilation.
Keywords: asthma; bronchoprotection; muscle mechanics; ultrastructure.
Copyright © 2015 the American Physiological Society.
Figures








References
-
- An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chitano P, Deng L, Dowell M, Eidelman DH, Fabry B, Fairbank NJ, Ford LE, Fredberg JJ, Gerthoffer WT, Gilbert SH, Gosens R, Gunst SJ, Halayko AJ, Ingram RH, Irvin CG, James AL, Janssen LJ, King GG, Knight DA, Lauzon AM, Lakser OJ, Ludwig MS, Lutchen KR, Maksym GN, Martin JG, Mauad T, McParland BE, Mijailovich SM, Mitchell HW, Mitchell RW, Mitzner W, Murphy TM, Paré PD, Pellegrino R, Sanderson MJ, Schellenberg RR, Seow CY, Silveira PS, Smith PG, Solway J, Stephens NL, Sterk PJ, Stewart AG, Tang DD, Tepper RS, Tran T, Wang L. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J 29: 834–860, 2007. - PMC - PubMed
-
- Chin LY, Bossé Y, Pascoe C, Hackett TL, Seow CY, Paré PD. Mechanical properties of asthmatic airway smooth muscle. Eur Respir J 40(1): 45–54, 2012. - PubMed
-
- Craig R, Smith R, Kendrick-Jones J. Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. Nature 302: 436–439, 1983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

