Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells
- PMID: 25306246
- PMCID: PMC4318247
- DOI: 10.1038/nbt.3048
Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells
Abstract
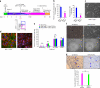
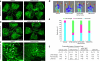
The ability to differentiate human pluripotent stem cells into endothelial cells with properties of cord-blood endothelial colony-forming cells (CB-ECFCs) may enable the derivation of clinically relevant numbers of highly proliferative blood vessel-forming cells to restore endothelial function in patients with vascular disease. We describe a protocol to convert human induced pluripotent stem cells (hiPSCs) or embryonic stem cells (hESCs) into cells similar to CB-ECFCs at an efficiency of >10(8) ECFCs produced from each starting pluripotent stem cell. The CB-ECFC-like cells display a stable endothelial phenotype with high clonal proliferative potential and the capacity to form human vessels in mice and to repair the ischemic mouse retina and limb, and they lack teratoma formation potential. We identify Neuropilin-1 (NRP-1)-mediated activation of KDR signaling through VEGF165 as a critical mechanism for the emergence and maintenance of CB-ECFC-like cells.
Figures


References
-
- Medina RJ, O'Neill CL, Humphreys MW, Gardiner TA, Stitt AW. Outgrowth endothelial cells: characterization and their potential for reversing ischemic retinopathy. Invest. Ophthalmol. Vis. Sci. 2010;51:5906–5913. - PubMed
-
- Moubarik C, et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev. 2011;7:208–220. - PubMed
-
- Ingram DA, et al. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–2786. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

