Smoothened (SMO) receptor mutations dictate resistance to vismodegib in basal cell carcinoma
- PMID: 25306392
- PMCID: PMC5528667
- DOI: 10.1016/j.molonc.2014.09.003
Smoothened (SMO) receptor mutations dictate resistance to vismodegib in basal cell carcinoma
Abstract
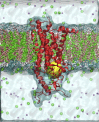
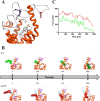
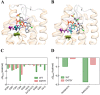
Basal cell carcinomas (BCCs) and a subset of medulloblastomas are characterized by loss-of-function mutations in the tumor suppressor gene, PTCH1. PTCH1 normally functions by repressing the activity of the Smoothened (SMO) receptor. Inactivating PTCH1 mutations result in constitutive Hedgehog pathway activity through uncontrolled SMO signaling. Targeting this pathway with vismodegib, a novel SMO inhibitor, results in impressive tumor regression in patients harboring genetic defects in this pathway. However, a secondary mutation in SMO has been reported in medulloblastoma patients following relapse on vismodegib to date. This mutation preserves pathway activity, but appears to confer resistance by interfering with drug binding. Here we report for the first time on the molecular mechanisms of resistance to vismodegib in two BCC cases. The first case, showing progression after 2 months of continuous vismodegib (primary resistance), exhibited the new SMO G497W mutation. The second case, showing a complete clinical response after 5 months of treatment and a subsequent progression after 11 months on vismodegib (secondary resistance), exhibited a PTCH1 nonsense mutation in both the pre- and the post-treatment specimens, and the SMO D473Y mutation in the post-treatment specimens only. In silico analysis demonstrated that SMO(G497W) undergoes a conformational rearrangement resulting in a partial obstruction of the protein drug entry site, whereas the SMO D473Y mutation induces a direct effect on the binding site geometry leading to a total disruption of a stabilizing hydrogen bond network. Thus, the G497W and D473Y SMO mutations may represent two different mechanisms leading to primary and secondary resistance to vismodegib, respectively.
Keywords: Basal cell carcinoma; Hedgehog pathway; PTCH1; Primary resistance; SMO; Secondary resistance; Vismodegib.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Andricioaei, I. , Karplus, M. , 2011. On the calculation of entropy from covariance matrices of the atomic fluctuations. J. Chem. Phys. 115, 6289–6292.
-
- Bayly, C.I. , Cieplak, P. , Cornell, W.D. , Kollman, P.A. , 1993. A well-behaved electrostatic potential based method using charge restraints for determining atom-centered charges: the RESP model. J. Phys. Chem. 97, 10269–10280.
-
- Berendsen, H.J.C. , Postma, J.P.M. , Van Gunsteren, W.F. , DiNola, A. , Haak, J.R. , 1984. Molecular-dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690.
-
- Besler, B.H. , Merz, K.M. , Kollman, P.A. , 1990. Atomic charges derived from semiempirical methods. J. Comput. Chem. 11, 431–439.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

