Cellular and Molecular Mediators of Intestinal Fibrosis
- PMID: 25306501
- PMCID: PMC5885809
- DOI: 10.1016/j.crohns.2014.09.008
Cellular and Molecular Mediators of Intestinal Fibrosis
Abstract
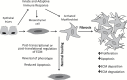
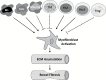
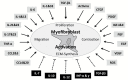
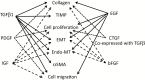
Intestinal fibrosis is a major complication of the inflammatory bowel diseases (IBD) and although inflammation is necessary for its development, it would appear that it plays a minor role in its progression as anti-inflammatory treatments in IBD do not prevent fibrosis once it has started. The processes that regulate fibrosis would thus appear to be distinct from those regulating inflammation and, therefore, a detailed understanding of these pathways is vital to the development of anti-fibrogenic strategies. There have been several recent reviews exploring what is known, and what remains unknown, about the development of intestinal fibrosis. This review is designed to add to this literature but with a focus on the cellular components that are involved in the development of fibrogenesis and the major molecular mediators that impact on these cells. The aim is to heighten the understanding of the factors involved in intestinal fibrogenesis so that detailed research can be encouraged in order to advance the processes that could lead to effective treatments.
Keywords: chemokine; extracellular matrix; fibroblast; growth factor; inflammatory bowel disease; interleukin; intestinal fibrosis; myofibroblast.
© 2015 Published on behalf of European Crohn’s and Colitis Organisation.
Figures

References
-
- Lawrance IC, Maxwell L, Doe W. Inflammation location, but not type, determines the increase in TGF-beta1 and IGF-1 expression and collagen deposition in IBD intestine. Inflamm Bowel Dis 2001;7:16–26. - PubMed
-
- Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 2002;8:244–50. - PubMed
-
- Longo WE, Virgo KS, Bahadursingh AN, Johnson FE. Patterns of disease and surgical treatment among United States veterans more than 50 years of age with ulcerative colitis. Am J Surg 2003;186:514–18. - PubMed
-
- Van Assche G, Geboes K, Rutgeerts P. Medical therapy for Crohn’s disease strictures. Inflamm Bowel Dis 2004;10:55–60. - PubMed
-
- Gearry RB, Richardson A, Frampton CMA, Collett JA, Burt MJ, Chapman BA, et al. High incidence of Crohn’s disease in Canterbury, New Zealand: Results of an epidemiologic study. Inflamm Bowel Dis 2006;12(10):936–943. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

