P-glycoprotein trafficking as a therapeutic target to optimize CNS drug delivery
- PMID: 25307213
- PMCID: PMC4301584
- DOI: 10.1016/bs.apha.2014.06.009
P-glycoprotein trafficking as a therapeutic target to optimize CNS drug delivery
Abstract
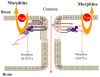
The primary function of the blood-brain barrier (BBB)/neurovascular unit is to protect the central nervous system (CNS) from potentially harmful xenobiotic substances and maintain CNS homeostasis. Restricted access to the CNS is maintained via a combination of tight junction proteins as well as a variety of efflux and influx transporters that limits the transcellular and paracellular movement of solutes. Of the transporters identified at the BBB, P-glycoprotein (P-gp) has emerged as the transporter that is the greatest obstacle to effective CNS drug delivery. In this chapter, we provide data to support intracellular protein trafficking of P-gp within cerebral capillary microvessels as a potential target for improved drug delivery. We show that pain-induced changes in P-gp trafficking are associated with changes in P-gp's association with caveolin-1, a key scaffolding/trafficking protein that colocalizes with P-gp at the luminal membrane of brain microvessels. Changes in colocalization with the phosphorylated and nonphosphorylated forms of caveolin-1, by pain, are accompanied by dynamic changes in the distribution, relocalization, and activation of P-gp "pools" between microvascular endothelial cell subcellular compartments. Since redox-sensitive processes may be involved in signaling disassembly of higher-order structures of P-gp, we feel that manipulating redox signaling, via specific protein targeting at the BBB, may protect disulfide bond integrity of P-gp reservoirs and control trafficking to the membrane surface, providing improved CNS drug delivery. The advantage of therapeutic drug "relocalization" of a protein is that the physiological impact can be modified, temporarily or long term, despite pathology-induced changes in gene transcription.
Keywords: ATP-binding cassette transporter; Blood–brain barrier; Drug delivery; Neurovascular unit; P-glycoprotein; Peripheral inflammatory pain; Protein trafficking; Reactive oxygen species; Redox-sensitive protein pools.
© 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures

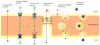



References
-
- Barakat S, Demeule M, Pilorget A, Regina A, Gingras D, Baggetto LG, et al. Modulation of p-glycoprotein function by caveolin-1 phosphorylation. J Neurochem. 2007;101:1–8. - PubMed
-
- Bendayan R, Lee G, Bendayan M. Functional expression and localization of P-glycoprotein at the blood brain barrier. Microsc Res Tech. 2002;57:365–380. - PubMed
-
- Betz AL, Firth JA, Goldstein GW. Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Research. 1980;192:17–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

