Increased post-induction intensification improves outcome in children and adolescents with a markedly elevated white blood cell count (≥200 × 10(9) /l) with T cell acute lymphoblastic leukaemia but not B cell disease: a report from the Children's Oncology Group
- PMID: 25308804
- PMCID: PMC4314336
- DOI: 10.1111/bjh.13160
Increased post-induction intensification improves outcome in children and adolescents with a markedly elevated white blood cell count (≥200 × 10(9) /l) with T cell acute lymphoblastic leukaemia but not B cell disease: a report from the Children's Oncology Group
Abstract
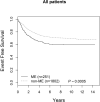
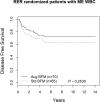
Children and adolescents presenting with a markedly elevated white blood cell (ME WBC) count (WBC ≥200 × 10(9) /l) comprise a unique subset of high-risk patients with acute lymphoblastic leukaemia (ALL). We evaluated the outcomes of the 251 patients (12% of the study population) with ME WBC treated on the Children's Cancer Group-1961 protocol. Patients were evaluated for early response to treatment by bone marrow morphology; those with a rapid early response were randomized to treatment regimens testing longer and stronger post-induction therapy. We found that ME WBC patients have a poorer outcome compared to those patients presenting with a WBC <200 × 10(9) /l (5-year event-free survival 62% vs. 73%, P = 0·0005). Longer duration of therapy worsened outcome for T cell ME WBC with a trend to poorer outcome in B-ALL ME WBC patients. Augmented therapy benefits T cell ME WBC patients, similar to the entire study cohort, however, there appeared to be no impact on survival for B-ALL ME WBC patients. ME WBC was not a prognostic factor for T cell patients. In patients with high risk features, B lineage disease in association with ME WBC has a negative impact on survival.
Keywords: acute lymphoblastic leukaemia; elevated white blood cell count; intensification; paediatric.
© 2014 John Wiley & Sons Ltd.
Figures












References
-
- Arico M, Valsecchi MG, Rizzari C, Barisone E, Biondi A, Casale F, Locatelli F, Lo Nigro L, Luciani M, Messina C, Micalizzi C, Parasole R, Pession A, Santoro N, Testi AM, Silvestri D, Basso G, Masera G, Conter V. Long-term results of the AIEOP-ALL-95 Trial for Childhood Acute Lymphoblastic Leukemia: insight on the prognostic value of DNA index in the framework of Berlin-Frankfurt-Muenster based chemotherapy. J Clin Oncol. 2008;26:283–289. - PubMed
-
- Asselin BL, Devidas M, Wang C, Pullen J, Borowitz MJ, Hutchison R, Lipshultz SE, Camitta BM. Effectiveness of high dose methotrexate in T-cell lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a randomized study by the Children's Oncology Group (POG 9404). Blood. 2011;118:874–883. - PMC - PubMed
-
- Dunsmore KP, Devidas M, Linda SB, Borowitz MJ, Winick N, Hunger SP, Carroll WL, Camitta BM. Pilot study of nelarabine in combination with intensive chemotherapy in high-risk T-cell acute lymphoblastic leukemia: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:2753–2759. - PMC - PubMed
-
- Gaynon PS, Steinherz PG, Bleyer WA, Ablin AR, Albo VC, Finklestein JZ, Grossman NJ, Novak LJ, Pyesmany AF, Reaman GH. Improved therapy for children with acute lymphoblastic leukemia and unfavorable presenting features: a follow-up report of the Childrens Cancer Group Study CCG-106. J Clin Oncol. 1993;11:2234–2242. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

