Edible bird's nest ameliorates oxidative stress-induced apoptosis in SH-SY5Y human neuroblastoma cells
- PMID: 25308934
- PMCID: PMC4210536
- DOI: 10.1186/1472-6882-14-391
Edible bird's nest ameliorates oxidative stress-induced apoptosis in SH-SY5Y human neuroblastoma cells
Abstract
Background: Parkinson's disease (PD) is the second most common neurodegenerative disorder affecting the senile population with manifestation of motor disability and cognitive impairment. Reactive oxygen species (ROS) is implicated in the progression of oxidative stress-related apoptosis and cell death of the midbrain dopaminergic neurons. Its interplay with mitochondrial functionality constitutes an important aspect of neuronal survival in the perspective of PD. Edible bird's nest (EBN) is an animal-derived natural food product made of saliva secreted by swiftlets from the Aerodamus genus. It contains bioactive compounds which might confer neuroprotective effects to the neurons. Hence this study aims to investigate the neuroprotective effect of EBN extracts in the neurotoxin-induced in vitro PD model.
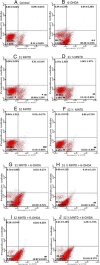
Methods: EBN was first prepared into pancreatin-digested crude extract and water extract. In vitro PD model was generated by exposing SH-SY5Y cells to neurotoxin 6-hydroxydopamine (6-OHDA). Cytotoxicity of the extracts on SH-SY5Y cells was tested using MTT assay. Then, microscopic morphological and nuclear examination, cell viability test and ROS assay were performed to assess the protective effect of EBN extracts against 6-OHDA-induced cellular injury. Apoptotic event was later analysed with Annexin V-propidium iodide flow cytometry. To understand whether the mechanism underlying the neuroprotective effect of EBN was mediated via mitochondrial or caspase-dependent pathway, mitochondrial membrane potential (MMP) measurement and caspase-3 quantification were carried out.
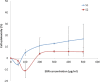
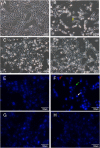
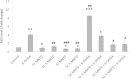
Results: Cytotoxicity results showed that crude EBN extract did not cause SH-SY5Y cell death at concentrations up to 75 μg/ml while the maximum non-toxic dose (MNTD) of water extract was double of that of crude extract. Morphological observation and nuclear staining suggested that EBN treatment reduced the level of 6-OHDA-induced apoptotic changes in SH-SY5Y cells. MTT study further confirmed that cell viability was better improved with crude EBN extract. However, water extract exhibited higher efficacy in ameliorating ROS build up, early apoptotic membrane phosphatidylserine externalization as well as inhibition of caspase-3 cleavage. None of the EBN treatment had any effect on MMP.
Conclusions: Current findings suggest that EBN extracts might confer neuroprotective effect against 6-OHDA-induced degeneration of dopaminergic neurons, particularly through inhibition of apoptosis. Thus EBN may be a viable nutraceutical option to protect against oxidative stress-related neurodegenerative disorders such as PD.
Figures


Similar articles
-
Anthraquinone from Edible Fungi Pleurotus ostreatus Protects Human SH-SY5Y Neuroblastoma Cells Against 6-Hydroxydopamine-Induced Cell Death-Preclinical Validation of Gene Knockout Possibilities of PARK7, PINK1, and SNCA1 Using CRISPR SpCas9.Appl Biochem Biotechnol. 2020 Jun;191(2):555-566. doi: 10.1007/s12010-019-03188-7. Epub 2019 Dec 9. Appl Biochem Biotechnol. 2020. PMID: 31820379
-
Neuroprotective effects of Liriope platyphylla extract against hydrogen peroxide-induced cytotoxicity in human neuroblastoma SH-SY5Y cells.BMC Complement Altern Med. 2015 Jun 9;15:171. doi: 10.1186/s12906-015-0679-3. BMC Complement Altern Med. 2015. PMID: 26054856 Free PMC article.
-
Evaluation of the neuroprotective potential of caffeic acid phenethyl ester in a cellular model of Parkinson's disease.Eur J Pharmacol. 2020 Sep 15;883:173342. doi: 10.1016/j.ejphar.2020.173342. Epub 2020 Jul 4. Eur J Pharmacol. 2020. PMID: 32634439
-
Edible Bird's Nest as a Potential Cognitive Enhancer.Front Neurol. 2022 May 6;13:865671. doi: 10.3389/fneur.2022.865671. eCollection 2022. Front Neurol. 2022. PMID: 35599726 Free PMC article. Review.
-
Edible Bird's Nest: Recent Updates and Industry Insights Based On Laboratory Findings.Front Pharmacol. 2021 Oct 1;12:746656. doi: 10.3389/fphar.2021.746656. eCollection 2021. Front Pharmacol. 2021. PMID: 34658881 Free PMC article. Review.
Cited by
-
The effectiveness of edible bird's nest in lowering VEGF, CD31, and PDGFR-β levels in diabetic retinopathy in rats with type 1 diabetes.Histol Histopathol. 2025 May;40(5):669-678. doi: 10.14670/HH-18-825. Epub 2024 Oct 1. Histol Histopathol. 2025. PMID: 39449415
-
The Anti-Viral and Anti-Inflammatory Properties of Edible Bird's Nest in Influenza and Coronavirus Infections: From Pre-Clinical to Potential Clinical Application.Front Pharmacol. 2021 May 7;12:633292. doi: 10.3389/fphar.2021.633292. eCollection 2021. Front Pharmacol. 2021. PMID: 34025406 Free PMC article. Review.
-
Edible Bird's Nest Prevents High Fat Diet-Induced Insulin Resistance in Rats.J Diabetes Res. 2015;2015:760535. doi: 10.1155/2015/760535. Epub 2015 Jul 27. J Diabetes Res. 2015. PMID: 26273674 Free PMC article.
-
The role of edible bird's nest and mechanism of averting lead acetate toxicity effect on rat uterus.Vet World. 2019 Jul;12(7):1013-1021. doi: 10.14202/vetworld.2019.1013-1021. Epub 2019 Jul 12. Vet World. 2019. PMID: 31528026 Free PMC article.
-
The improvement effects of edible bird's nest on proliferation and activation of B lymphocyte and its antagonistic effects on immunosuppression induced by cyclophosphamide.Drug Des Devel Ther. 2016 Jan 21;10:371-81. doi: 10.2147/DDDT.S88193. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 26855562 Free PMC article.
References
-
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. - DOI - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1472-6882/14/391/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

