Directional interactions and cooperativity between mechanosensitive membrane proteins
- PMID: 25309021
- PMCID: PMC4193682
- DOI: 10.1209/0295-5075/101/68002
Directional interactions and cooperativity between mechanosensitive membrane proteins
Abstract
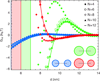
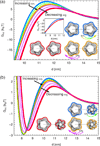
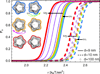
While modern structural biology has provided us with a rich and diverse picture of membrane proteins, the biological function of membrane proteins is often influenced by the mechanical properties of the surrounding lipid bilayer. Here we explore the relation between the shape of membrane proteins and the cooperative function of membrane proteins induced by membrane-mediated elastic interactions. For the experimental model system of mechanosensitive ion channels we find that the sign and strength of elastic interactions depend on the protein shape, yielding distinct cooperative gating curves for distinct protein orientations. Our approach predicts how directional elastic interactions affect the molecular structure, organization, and biological function of proteins in crowded membranes.
Figures

Similar articles
-
Connection between oligomeric state and gating characteristics of mechanosensitive ion channels.PLoS Comput Biol. 2013;9(5):e1003055. doi: 10.1371/journal.pcbi.1003055. Epub 2013 May 16. PLoS Comput Biol. 2013. PMID: 23696720 Free PMC article.
-
Lipid bilayer mediates ion-channel cooperativity in a model of hair-cell mechanotransduction.Proc Natl Acad Sci U S A. 2017 Dec 19;114(51):E11010-E11019. doi: 10.1073/pnas.1713135114. Epub 2017 Dec 7. Proc Natl Acad Sci U S A. 2017. PMID: 29217640 Free PMC article.
-
Bilayer-thickness-mediated interactions between integral membrane proteins.Phys Rev E. 2016 Apr;93:042410. doi: 10.1103/PhysRevE.93.042410. Epub 2016 Apr 18. Phys Rev E. 2016. PMID: 27176332
-
Mechanosensitivity of ion channels based on protein-lipid interactions.J R Soc Interface. 2010 Jun 6;7 Suppl 3(Suppl 3):S307-20. doi: 10.1098/rsif.2010.0095.focus. Epub 2010 Mar 31. J R Soc Interface. 2010. PMID: 20356872 Free PMC article. Review.
-
Lipid-protein interactions: Lessons learned from stress.Biochim Biophys Acta. 2015 Sep;1848(9):1744-56. doi: 10.1016/j.bbamem.2015.04.012. Epub 2015 Apr 25. Biochim Biophys Acta. 2015. PMID: 25922225 Review.
Cited by
-
New Continuum Approaches for Determining Protein-Induced Membrane Deformations.Biophys J. 2017 May 23;112(10):2159-2172. doi: 10.1016/j.bpj.2017.03.040. Biophys J. 2017. PMID: 28538153 Free PMC article.
-
Pre-transition effects mediate forces of assembly between transmembrane proteins.Elife. 2016 Feb 24;5:e13150. doi: 10.7554/eLife.13150. Elife. 2016. PMID: 26910009 Free PMC article.
-
The role of membrane-mediated interactions in the assembly and architecture of chemoreceptor lattices.PLoS Comput Biol. 2014 Dec 11;10(12):e1003932. doi: 10.1371/journal.pcbi.1003932. eCollection 2014 Dec. PLoS Comput Biol. 2014. PMID: 25503274 Free PMC article.
-
Decisive structural elements in water and ion permeation through mechanosensitive channels of large conductance: insights from molecular dynamics simulation.RSC Adv. 2022 Jun 16;12(28):17803-17816. doi: 10.1039/d2ra02284b. eCollection 2022 Jun 14. RSC Adv. 2022. PMID: 35765322 Free PMC article.
-
Continuum descriptions of membranes and their interaction with proteins: Towards chemically accurate models.Biochim Biophys Acta. 2016 Jul;1858(7 Pt B):1619-34. doi: 10.1016/j.bbamem.2016.02.003. Epub 2016 Feb 4. Biochim Biophys Acta. 2016. PMID: 26853937 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
