GABAA Receptor Expression in the Forebrain of Ataxic Rolling Nagoya Mice
- PMID: 25309056
- PMCID: PMC4191822
- DOI: 10.4172/1234-3425.1000198
GABAA Receptor Expression in the Forebrain of Ataxic Rolling Nagoya Mice
Abstract
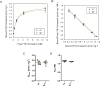
The human CACNA1A gene encodes the pore-forming α1 subunit of CaV2.1 (P/Q-type) calcium channels and is the locus for several neurological disorders, including episodic ataxia type 2 (EA2), spinocerebellar ataxia type 6 (SCA6) and Familial Hemiplegic Migraine type 1 (FHM1). Several spontaneous mouse Cacna1a mutant strains exist, among them Rolling Nagoya (tgrol), carrying the R1262G point mutation in the mouse Cacna1a gene. tgrol mice display a phenotype of severe gait ataxia and motor dysfunction of the hind limbs. At the functional level, the R1262G mutation results in a positive shift of the activation voltage of the CaV2.1 channel and reduced current density. γ-Aminobutyric acid type A (GABAA) receptor subunit expression depends critically on neuronal calcium influx, and GABAA receptor dysfunction has previously been described for the cerebellum of tgrol and other ataxic Cacna1a mutant mice. Given the expression pattern of CaV2.1, it was hypothesized that calcium dysregulation in tgrol might affect GABAA receptor expression in the forebrain. Herein, functional GABAA receptors in the forebrain of tgrol mice were quantified and pharmacologically dissociated using [3H] radioligand binding. No gross changes to functional GABAA receptors were identified. Future cell type-specific analyses are required to identify possible cortical contributions to the psychomotor phenotype of tgrol mice.
Keywords: Ataxia; CaV2.1; Cacan1a; Calcium; Gamma aminobutyric receptor type A; Motor dysfunction; P/Q-type calcium channel; Pharmacology; Rolling Nagoya.
Figures


Similar articles
-
Differential cerebellar GABAA receptor expression in mice with mutations in CaV2.1 (P/Q-type) calcium channels.Neuroscience. 2015 Sep 24;304:198-208. doi: 10.1016/j.neuroscience.2015.07.044. Epub 2015 Jul 21. Neuroscience. 2015. PMID: 26208839 Free PMC article.
-
Early onset, non fluctuating spinocerebellar ataxia and a novel missense mutation in CACNA1A gene.J Neurol Sci. 2006 Feb 15;241(1-2):13-7. doi: 10.1016/j.jns.2005.10.007. Epub 2005 Dec 2. J Neurol Sci. 2006. PMID: 16325861
-
Differential regulation of Purkinje cell dendritic spines in rolling mouse Nagoya (tg/tg), P/Q type calcium channel (α1(A)/Ca(v)2.1) mutant.Anat Cell Biol. 2010 Sep;43(3):211-7. doi: 10.5115/acb.2010.43.3.211. Epub 2010 Sep 30. Anat Cell Biol. 2010. PMID: 21212861 Free PMC article.
-
CaV2.1 channelopathies.Pflugers Arch. 2010 Jul;460(2):375-93. doi: 10.1007/s00424-010-0802-8. Epub 2010 Mar 4. Pflugers Arch. 2010. PMID: 20204399 Review.
-
Molecular mechanism of Spinocerebellar Ataxia type 6: glutamine repeat disorder, channelopathy and transcriptional dysregulation. The multifaceted aspects of a single mutation.Front Cell Neurosci. 2015 Feb 16;9:36. doi: 10.3389/fncel.2015.00036. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25762895 Free PMC article. Review.
References
-
- Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. - PubMed
-
- Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 1997;15:62–69. - PubMed
-
- Jouvenceau A, Eunson LH, Spauschus A, Ramesh V, Zuberi SM, et al. Human epilepsy associated with dysfunction of the brain P/Q-type calcium channel. Lancet. 2001;358:801–807. - PubMed
-
- Imbrici P, Jaffe SL, Eunson LH, Davies NP, Herd C, et al. Dysfunction of the brain calcium channel CaV2.1 in absence epilepsy and episodic ataxia. Brain. 2004;127:2682–2692. - PubMed
-
- GREEN MC, SIDMAN RL. Tottering–a neuromusclar mutation in the mouse. And its linkage with oligosyndacylism. J Hered. 1962;53:233–237. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
