Crystal structure of (E)-1(anthracen-9-ylmethylidene)[2-(morpholin-4-yl)eth-yl]amine
- PMID: 25309217
- PMCID: PMC4186061
- DOI: 10.1107/S1600536814018807
Crystal structure of (E)-1(anthracen-9-ylmethylidene)[2-(morpholin-4-yl)eth-yl]amine
Abstract
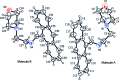
The title compound, C21H22N2O, crystallizes with two independent mol-ecules in the asymmetric unit. In both mol-ecules, the anthracene ring systems are almost planar, with maximum deviations of 0.071 (8) and 0.028 (7) Å, and make dihedral angles of 73.4 (2) and 73.3 (2)° with the least-squares planes formed by the four C atoms of the morpholine rings, which adopt a chair conformation. An intra-molecular C-H⋯π inter-action occurs. In the crystal, the packing is stabilized by weak C-H⋯O hydrogen bonds, which connect pairs of molecules into parallel to the c axis, and C-H⋯π inter-actions.
Keywords: C—H⋯π interactions; Schiff bases; anthracene; crystal structure; methanimine; morpholine.
Figures

References
-
- Dhar, D. N. & Taploo, C. L. (1982). J. Sci. Ind. Res. 41, 501–506.
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
-
- Gerdemann, C., Eicken, C. & Krebs, B. (2002). Acc. Chem. Res. 35, 183–191. - PubMed
-
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. - PubMed
-
- Solomon, E. I. & Lowery, M. D. (1993). Science, 259, 1575–1581. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
