Crystal structure of (Z)-2,3-di-chloro-1,4-bis-(4-meth-oxy-phen-yl)but-2-ene-1,4-dione
- PMID: 25309219
- PMCID: PMC4186131
- DOI: 10.1107/S1600536814018790
Crystal structure of (Z)-2,3-di-chloro-1,4-bis-(4-meth-oxy-phen-yl)but-2-ene-1,4-dione
Abstract
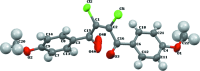
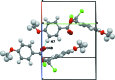
The title compound, C18H14Cl2O4, adopts a Z conformation around the cental C=C bond. The two aromatic rings of the mol-ecule are nearly perpendicular to each other, with a dihedral angle between of 86.22 (14)°. The meth-oxy substituents lie close to the plane of the attached benzene rings. The C(ar)-C(ar)-O-C(Me) torsion angles are -2.4 (7) and 7.5 (6)°. Weak C-H⋯O inter-actions link the mol-ecules forming a three-dimensional network. The crystal packing also displays short [3.160 (3) Å] Cl⋯O halogen-bonding contacts between mol-ecules related by the screw axis. The structure exhibits disorder of one carbonyl O atom with a refined occupancy ratio of 0.21 (6):0.79 (6).
Keywords: 2,3-dichlorobut-2-ene-1,4-dione; CuCl/bpy; crystal structure; dichloromethyl radical; stereoselectivity; trichloromethyl groups.
Figures

References
-
- Bruker (2000). SMART, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Clark, A. J. (2002). Chem. Soc. Rev. 31, 1–11. - PubMed
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
-
- Kurosawa, K. & Yamaguchi, K. (1981). Bull. Chem. Soc. Jpn, 54, 1757–1760.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
