Astrocytosis in parkinsonism: considering tripartite striatal synapses in physiopathology?
- PMID: 25309435
- PMCID: PMC4174038
- DOI: 10.3389/fnagi.2014.00258
Astrocytosis in parkinsonism: considering tripartite striatal synapses in physiopathology?
Abstract
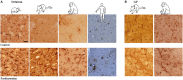
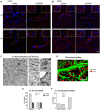
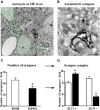
The current concept of basal ganglia organization and function in physiological and pathophysiological conditions excludes the most numerous cells in the brain, i.e., the astrocytes, present with a ratio of 10:1 neuron. Their role in neurodegenerative condition such as Parkinson's disease (PD) remains to be elucidated. Before embarking into physiological investigations of the yet-to-be-identified "tripartite" synapses in the basal ganglia in general and the striatum in particular, we therefore characterized anatomically the PD-related modifications in astrocytic morphology, the changes in astrocytic network connections and the consequences on the spatial relationship between astrocytic processes and asymmetric synapses in normal and PD-like conditions in experimental and human PD. Our results unravel a dramatic regulation of striatal astrocytosis supporting the hypothesis of a key role in (dys) regulating corticostriatal transmission. Astrocytes and their various properties might thus represent a therapeutic target in PD.
Keywords: astrocyte; dopamine; human; immunohistochemistry; medium spiny neuron; monkey; mouse; rat.
Figures

References
-
- Ball K. K., Gandhi G. K., Thrash J., Cruz N. F., Dienel G. A. (2007). Astrocytic connexin distributions and rapid, extensive dye transfer via gap junctions in the inferior colliculus: implications for [(14)C]glucose metabolite trafficking. J. Neurosci. Res. 85, 3267–3283 10.1002/jnr.21376 - DOI - PMC - PubMed
-
- Berthet A., Porras G., Doudnikoff E., Stark H., Cador M., Bezard E., et al. (2009). Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of L-DOPA-induced dyskinesia. J. Neurosci. 29, 4829–4835 10.1523/jneurosci.5884-08.2009 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

