A possible link between BDNF and mTOR in control of food intake
- PMID: 25309497
- PMCID: PMC4174734
- DOI: 10.3389/fpsyg.2014.01093
A possible link between BDNF and mTOR in control of food intake
Abstract
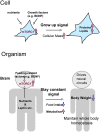
Food intake is intricately regulated by glucose, amino acids, hormones, neuropeptides, and trophic factors through a neural circuit in the hypothalamus. Brain-derived neurotrophic factor (BDNF), the most prominent neurotrophic factor in the brain, regulates differentiation, maturation, and synaptic plasticity throughout life. Among its many roles, BDNF exerts an anorexigenic function in the brain. However, the intracellular signaling induced by BDNF to control food intake is not fully understood. One candidate for the molecule involved in transducing the anorexigenic activity of BDNF is the mammalian target of rapamycin (mTOR). mTOR senses extracellular amino acids, glucose, growth factors, and neurotransmitters, and regulates anabolic reactions response to these signals. Activated mTOR increases protein and lipid synthesis and inhibits protein degradation. In the hypothalamus, mTOR activation is thought to reduce food intake. Here we summarize recent findings regarding BDNF- and mTOR-mediated feeding control, and propose a link between these molecules in eating behavior.
Keywords: AMPK; BDNF; body weight; food intake regulation; mTOR; mTORC1; nutrients.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

