Evolution and development of cell walls in cereal grains
- PMID: 25309555
- PMCID: PMC4161051
- DOI: 10.3389/fpls.2014.00456
Evolution and development of cell walls in cereal grains
Abstract
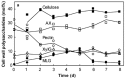
The composition of cell walls in cereal grains and other grass species differs markedly from walls in seeds of other plants. In the maternal tissues that surround the embryo and endosperm of the grain, walls contain higher levels of cellulose and in many cases are heavily lignified. This may be contrasted with walls of the endosperm, where the amount of cellulose is relatively low, and the walls are generally not lignified. The low cellulose and lignin contents are possible because the walls of the endosperm perform no load-bearing function in the mature grain and indeed the low levels of these relatively intractable wall components are necessary because they allow rapid degradation of the walls following germination of the grain. The major non-cellulosic components of endosperm walls are usually heteroxylans and (1,3;1,4)-β-glucans, with lower levels of xyloglucans, glucomannans, and pectic polysaccharides. Pectic polysaccharides and xyloglucans are the major non-cellulosic wall constituents in most dicot species, in which (1,3;1,4)-β-glucans are usually absent and heteroxylans are found at relatively low levels. Thus, the "core" non-cellulosic wall polysaccharides in grain of the cereals and other grasses are the heteroxylans and, more specifically, arabinoxylans. The (1,3;1,4)-β-glucans appear in the endosperm of some grass species but are essentially absent from others; they may constitute from zero to more than 45% of the cell walls of the endosperm, depending on the species. It is clear that in some cases these (1,3;1,4)-β-glucans function as a major store of metabolizable glucose in the grain. Cereal grains and their constituent cell wall polysaccharides are centrally important as a source of dietary fiber in human societies and breeders have started to select for high levels of non-cellulosic wall polysaccharides in grain. To meet end-user requirements, it is important that we understand cell wall biology in the grain both during development and following germination.
Keywords: (1,3;1,4)-β-glucan; arabinoxylans; biosynthesis; cellulose; evolution; non-cellulosic polysaccharides.
Figures



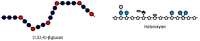
References
-
- Bacic A., Stone B. A. (1981). Chemistry and organization of aleurone cell wall components from wheat and barley. Funct. Plant Biol. 8 475–495 10.1071/PP9810475 - DOI
-
- Becraft P. W., Asuncion-Crabb Y. (2000). Positional cues specify and maintain aleurone cell fate in maize endosperm development. Development 127 4039–4048 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

