Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury
- PMID: 25309723
- PMCID: PMC4193961
- DOI: 10.1186/2045-824X-6-21
Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury
Abstract
Background: The origins of neointimal smooth muscle cells that arise following vascular injury remains controversial. Studies have suggested that these cells may arise from previously differentiated medial vascular smooth muscle cells, resident stem cells or blood born progenitors. In the current study we examined the contribution of the previously differentiated vascular smooth muscle cells to the neointima that forms following carotid artery ligation.
Methods: We utilized transgenic mice harboring a cre recombinase-dependent reporter gene (mTmG). These mice express membrane targeted tandem dimer Tomato (mTomato) prior to cre-mediated excision and membrane targeted EGFP (mEGFP) following excision. The mTmG mice were crossed with transgenic mice expressing either smooth muscle myosin heavy chain (Myh11) or smooth muscle α-actin (Acta2) driven tamoxifen regulated cre recombinase. Following treatment of adult mice with tamoxifen these mice express mEGFP exclusively in differentiated smooth muscle cells. Subsequently vascular injury was induced in the mice by carotid artery ligation and the contribution of mEGFP positive cells to the neointima determined.
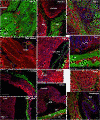
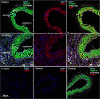
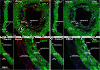
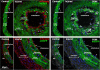
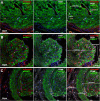
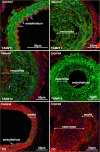
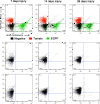
Results: Analysis of the cellular composition of the neointima that forms following injury revealed that mEGFP positive cells derived from either Mhy11 or Acta2 tagged medial vascular smooth muscle cells contribute to the majority of neointima formation (79 ± 17% and 81 ± 12%, respectively).
Conclusion: These data demonstrate that the majority of the neointima that forms following carotid ligation is derived from previously differentiated medial vascular smooth muscle cells.
Keywords: Neointima; Smooth muscle myosin; Smooth muscle α-actin; Vascular smooth muscle.
Figures




References
-
- Iwata H, Manabe I, Fujiu K, Yamamoto T, Takeda N, Eguchi K, Furuya A, Kuro-o M, Sata M, Nagai R. Bone marrow-derived cells contribute to vascular inflammation but do not differentiate into smooth muscle cell lineages. Circulation. 2010;122:2048–2057. doi: 10.1161/CIRCULATIONAHA.110.965202. - DOI - PubMed
-
- Nemenoff RA, Horita H, Ostriker AC, Furgeson SB, Simpson PA, VanPutten V, Crossno J, Offermanns S, Weiser-Evans MC. SDF-1alpha induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2011;31:1300–1308. doi: 10.1161/ATVBAHA.111.223701. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

