Mode of action of lactoperoxidase as related to its antimicrobial activity: a review
- PMID: 25309750
- PMCID: PMC4182067
- DOI: 10.1155/2014/517164
Mode of action of lactoperoxidase as related to its antimicrobial activity: a review
Abstract
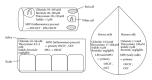
Lactoperoxidase is a member of the family of the mammalian heme peroxidases which have a broad spectrum of activity. Their best known effect is their antimicrobial activity that arouses much interest in in vivo and in vitro applications. In this context, the proper use of lactoperoxidase needs a good understanding of its mode of action, of the factors that favor or limit its activity, and of the features and properties of the active molecules. The first part of this review describes briefly the classification of mammalian peroxidases and their role in the human immune system and in host cell damage. The second part summarizes present knowledge on the mode of action of lactoperoxidase, with special focus on the characteristics to be taken into account for in vitro or in vivo antimicrobial use. The last part looks upon the characteristics of the active molecule produced by lactoperoxidase in the presence of thiocyanate and/or iodide with implication(s) on its antimicrobial activity.
Figures








References
-
- O'Brien PJ. Peroxidases. Chemico-Biological Interactions. 2000;129(1-2):113–139. - PubMed
-
- Jantschko W, Furtmüller PG, Allegra M, et al. Redox intermediates of plant and mammalian peroxidases: a comparative transient-kinetic study of their reactivity toward indole derivatives. Archives of Biochemistry and Biophysics. 2002;398(1):12–22. - PubMed
-
- Kimura S, Ikeda-Saito M. Human myeloperoxidase and thyroid peroxidase, two enzymes with separate and distinct physiological functions, are evolutionarily related members of the same gene family. Proteins: Structure, Function and Genetics. 1988;3(2):113–120. - PubMed
-
- Battistuzzi G, Bellei M, Bortolotti CA, Sola M. Redox properties of heme peroxidases. Archives of Biochemistry and Biophysics. 2010;500(1):21–36. - PubMed
-
- Zamocky M, Jakopitsch C, Furtmüller PG, Dunand C, Obinger C. The peroxidase-cyclooxygenase superfamily: reconstructed evolution of critical enzymes of the innate immune system. Proteins: Structure, Function and Genetics. 2008;72(2):589–605. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

