A sulfated nanofibrous mesh supporting the osteogenic differentiation of periosteum-derived cells
- PMID: 25309819
- PMCID: PMC4193908
- DOI: 10.1166/jbt.2013.1103
A sulfated nanofibrous mesh supporting the osteogenic differentiation of periosteum-derived cells
Abstract
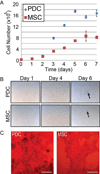
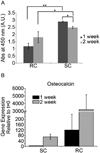
The periosteum is a thin fibrous membrane covering the surface of long bone and is known to play a critical role in bone development and adult bone fracture healing. Loss or damage of the periosteum tissue during traumatic long bone injuries can lead to retarded healing of bone graft-mediated repair. The regenerative potential of periosteum-derived progenitor cells (PDCs) has inspired their use as an alternative to bone marrow-derived mesenchymal stromal cells (MSCs) to augment scaffold-assisted bone repair. In this study, we first demonstrated that PDCs isolated from adult rat long bone exhibited innate advantages over bone marrow-derived MSCs in terms of faster proliferation and more potent osteogenic differentiation upon induction in plastic-adherent culture. Further, we examined the potential of two electrospun nanofibrous meshes, an uncharged regenerated cellulose mesh and a sulfated mesh, to support the attachment and osteogenic differentiation of PDCs. We showed that both nanofibrous meshes were able to support the attachment and proliferation of PDCs and MSCs alike, with the sulfated mesh enabling significantly higher seeding efficiency than the cellulose mesh. Both meshes were also able to support the osteogenic differentiation of adherent PDCs upon induction by osteogenic media, with the sulfated mesh facilitating more potent mineral deposition by adherent PDCs. Our study supports the sulfated nanofibrous mesh as a promising synthetic periosteal membrane for the delivery of exogenous PDCs to augment bone healing.
Figures


References
-
- Bonzani IC, George JH, Stevens MM. Curr Opin Chem Biol. 2006;10:568. - PubMed
-
- Michal Crha AN, Srnec Robert, Janovec Jan, Stehlik Ladislav, Rauser Petr, Urbanova Lucie, Planka Ladislav, jancar Josef, Amler Evzen. Acta Vet. BRNO. 2009;78:635.
-
- Mauney JR, Volloch V, Kaplan DL. Tissue Eng. 2005;11:787. - PubMed
-
- Lutolf MP, Hubbell JA. Nat Biotechnol. 2005;23:47. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
